Preparation of the membrane-permeant biarsenicals FlAsH-EDT2 and ReAsH-EDT2 for fluorescent labeling of tetracysteine-tagged proteins
- PMID: 18772880
- PMCID: PMC2843588
- DOI: 10.1038/nprot.2008.144
Preparation of the membrane-permeant biarsenicals FlAsH-EDT2 and ReAsH-EDT2 for fluorescent labeling of tetracysteine-tagged proteins
Abstract
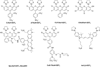
The membrane-permeant fluorogenic biarsenicals FlAsH-EDT(2) and ReAsH-EDT(2) can be prepared in good yields by a straightforward two-step procedure from the inexpensive precursor dyes fluorescein and resorufin, respectively. Handling of toxic reagents such as arsenic trichloride is minimized so the synthesis can be carried out in a typical chemistry laboratory, usually taking about 2-3 d. A wide range of other biarsenical reagents and intermediates that also bind to tetracysteine-tagged (CysCysProGlyCysCys) proteins can be prepared similarly using this general procedure.
Figures


References
-
- Tsien RY. The green fluorescent protein. Annu. Rev Biochem. 1998;67:509–544. - PubMed
-
- Giepmans BN, Adams SR, Ellisman MH, Tsien RY. The fluorescent toolbox for assessing protein location and function. Science. 2006;312:217–224. - PubMed
-
- Griffin BA, Adams SR, Tsien RY. Specific covalent labeling of recombinant protein molecules inside live cells. Science. 1998;281:269–272. - PubMed
-
- Adams SR, et al. New biarsenical ligands and tetracysteine motifs for protein labeling in vitro and in vivo: synthesis and biological applications. J. Am. Chem. Soc. 2002;124:6063–6076. - PubMed
-
- Martin BR, Giepmans BN, Adams SR, Tsien RY. Mammalian cell-based optimization of the biarsenical-binding tetracysteine motif for improved fluorescence and affinity. Nat. Biotechnol. 2005;23:1308–1314. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

