Serum microRNAs are promising novel biomarkers
- PMID: 18773077
- PMCID: PMC2519789
- DOI: 10.1371/journal.pone.0003148
Serum microRNAs are promising novel biomarkers
Abstract
Background: Circulating nucleic acids (CNAs) offer unique opportunities for early diagnosis of clinical conditions. Here we show that microRNAs, a family of small non-coding regulatory RNAs involved in human development and pathology, are present in bodily fluids and represent new effective biomarkers.
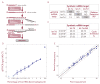
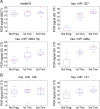
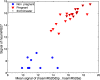
Methods and results: After developing protocols for extracting and quantifying microRNAs in serum and other body fluids, the serum microRNA profiles of several healthy individuals were determined and found to be similar, validating the robustness of our methods. To address the possibility that the abundance of specific microRNAs might change during physiological or pathological conditions, serum microRNA levels in pregnant and non pregnant women were compared. In sera from pregnant women, microRNAs associated with human placenta were significantly elevated and their levels correlated with pregnancy stage.
Conclusions and significance: Considering the central role of microRNAs in development and disease, our results highlight the medically relevant potential of determining microRNA levels in serum and other body fluids. Thus, microRNAs are a new class of CNAs that promise to serve as useful clinical biomarkers.
Conflict of interest statement
Figures


References
-
- Kopreski MS, Benko FA, Gocke DC. Circulating RNA as a tumor marker: detection of 5T4 mRNA in breast and lung cancer patient serum. Ann N Y Acad Sci. 2001;945:172–178. - PubMed
-
- Lo YM, Tsui NB, Chiu RW, Lau TK, Leung TN, et al. Plasma placental RNA allelic ratio permits noninvasive prenatal chromosomal aneuploidy detection. Nat Med. 2007;13:218–223. - PubMed
-
- Swarup V, Rajeswari RM. Circulating (cell-free) nucleic acids–a promising, non-invasive tool for early detection of several human diseases. FEBS Lett. 2007;581:795–799. - PubMed
-
- Pheasant M, Mattick SJ. Raising the estimate of functional human sequences. Genome Res. 2007;17:1245–1253. - PubMed
-
- Soifer HS, Rossi JJ, Saetrom P. MicroRNAs in Disease and Potential Therapeutic Applications. Mol Ther 2007 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

