Targeted destruction of photosensitive retinal ganglion cells with a saporin conjugate alters the effects of light on mouse circadian rhythms
- PMID: 18773079
- PMCID: PMC2519834
- DOI: 10.1371/journal.pone.0003153
Targeted destruction of photosensitive retinal ganglion cells with a saporin conjugate alters the effects of light on mouse circadian rhythms
Abstract
Non-image related responses to light, such as the synchronization of circadian rhythms to the day/night cycle, are mediated by classical rod/cone photoreceptors and by a small subset of retinal ganglion cells that are intrinsically photosensitive, expressing the photopigment, melanopsin. This raises the possibility that the melanopsin cells may be serving as a conduit for photic information detected by the rods and/or cones. To test this idea, we developed a specific immunotoxin consisting of an anti-melanopsin antibody conjugated to the ribosome-inactivating protein, saporin. Intravitreal injection of this immunotoxin results in targeted destruction of melanopsin cells. We find that the specific loss of these cells in the adult mouse retina alters the effects of light on circadian rhythms. In particular, the photosensitivity of the circadian system is significantly attenuated. A subset of animals becomes non-responsive to the light/dark cycle, a characteristic previously observed in mice lacking rods, cones, and functional melanopsin cells. Mice lacking melanopsin cells are also unable to show light induced negative masking, a phenomenon known to be mediated by such cells, but both visual cliff and light/dark preference responses are normal. These data suggest that cells containing melanopsin do indeed function as a conduit for rod and/or cone information for certain non-image forming visual responses. Furthermore, we have developed a technique to specifically ablate melanopsin cells in the fully developed adult retina. This approach can be applied to any species subject to the existence of appropriate anti-melanopsin antibodies.
Conflict of interest statement
Figures
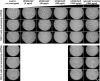
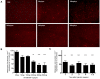
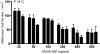



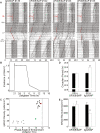

References
-
- Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. - PubMed
-
- Gooley JJ, Lu J, Chou TC, Scammell TE, Saper CB. Melanopsin in cells of origin of the retinohypothalamic tract. Nat Neurosci. 2001;4:1165. - PubMed
-
- Yoshimura T, Nishio M, Goto M, Ebihara S. Differences in circadian photosensitivity between retinally degenerate CBA/J mice ( rd/rd ) and normal CBA/N mice (+/+). Journal of Biological Rhythms. 1994;9:51–60. - PubMed
-
- Foster RG, Provencio I, Hudson D, Fiske S, De Grip W, et al. Circadian photoreception in the retinally degenerate mouse (rd/rd). J Comp Physiol [A] 1991;169:39–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

