Neuroprotective effect of transplanted human embryonic stem cell-derived neural precursors in an animal model of multiple sclerosis
- PMID: 18773082
- PMCID: PMC2522282
- DOI: 10.1371/journal.pone.0003145
Neuroprotective effect of transplanted human embryonic stem cell-derived neural precursors in an animal model of multiple sclerosis
Abstract
Background: Multiple sclerosis (MS) is an immune mediated demyelinating disease of the central nervous system (CNS). A potential new therapeutic approach for MS is cell transplantation which may promote remyelination and suppress the inflammatory process.
Methods: We transplanted human embryonic stem cells (hESC)-derived early multipotent neural precursors (NPs) into the brain ventricles of mice induced with experimental autoimmune encephalomyelitis (EAE), the animal model of MS. We studied the effect of the transplanted NPs on the functional and pathological manifestations of the disease.
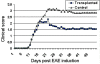
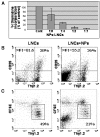
Results: Transplanted hESC-derived NPs significantly reduced the clinical signs of EAE. Histological examination showed migration of the transplanted NPs to the host white matter, however, differentiation to mature oligodendrocytes and remyelination were negligible. Time course analysis of the evolution and progression of CNS inflammation and tissue injury showed an attenuation of the inflammatory process in transplanted animals, which was correlated with the reduction of both axonal damage and demyelination. Co-culture experiments showed that hESC-derived NPs inhibited the activation and proliferation of lymph node-derived T cells in response to nonspecific polyclonal stimuli.
Conclusions: The therapeutic effect of transplantation was not related to graft or host remyelination but was mediated by an immunosuppressive neuroprotective mechanism. The attenuation of EAE by hESC-derived NPs, demonstrated here, may serve as the first step towards further developments of hESC for cell therapy in MS.
Conflict of interest statement
Figures




References
-
- Gironi M, Bergami A, Brambilla E, Ruffini F, Furlan R, et al. Immunological markers in multiple sclerosis. Neurol Sci. 2000;21(4 Suppl 2):S871–875. - PubMed
-
- Hemmer B, Cepok S, Nessler S, Sommer N. Pathogenesis of multiple sclerosis: an update on immunology. Curr Opin Neurol. 2002;15:227–231. - PubMed
-
- Lassmann H. Mechanisms of demyelination and tissue destruction in multiple sclerosis. Clin Neurol Neurosurg. 2002;104:168–171. - PubMed
-
- Franklin RJ. Why does remyelination fail in multiple sclerosis? Nat Rev Neurosci. 2002;3:705–714. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

