Rapid morphological brain abnormalities during acute methamphetamine intoxication in the rat: an experimental study using light and electron microscopy
- PMID: 18773954
- PMCID: PMC2638124
- DOI: 10.1016/j.jchemneu.2008.08.002
Rapid morphological brain abnormalities during acute methamphetamine intoxication in the rat: an experimental study using light and electron microscopy
Abstract
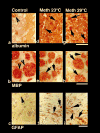
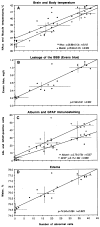
This study describes morphological abnormalities of brain cells during acute methamphetamine (METH) intoxication in the rat and demonstrates the role of hyperthermia, disruption of the blood-brain barrier (BBB) and edema in their development. Rats with chronically implanted brain, muscle and skin temperature probes and an intravenous (i.v.) catheter were exposed to METH (9 mg/kg) at standard (23 degrees C) and warm (29 degrees C) ambient temperatures, allowing for the observation of hyperthermia ranging from mild to pathological (38-42 degrees C). When brain temperature peaked or reached a level suggestive of possible lethality (>41.5 degrees C), rats were injected with Evans blue (EB), rapidly anesthetized, perfused, and their brains were taken for further analyses. Four brain areas (cortex, hippocampus, thalamus and hypothalamus) were analyzed for EB extravasation, water and electrolyte (Na(+), K(+), Cl(-)) contents, immunostained for albumin and glial fibrillary acidic protein (GFAP), and examined for neuronal, glial and axonal alterations using standard light and electron microscopy. These examinations revealed profound abnormalities in neuronal, glial, and endothelial cells, which were stronger with METH administered at 29 degrees C than 23 degrees C and tightly correlated with brain and body hyperthermia. These changes had some structural specificity, but in each structure they tightly correlated with increases in EB levels, the numbers of albumin-positive cells, and water and ion contents, suggesting leakage of the BBB, acutely developing brain edema, and serious shifts in brain ion homeostasis as leading factors underlying brain abnormalities. While most of these acute structural and functional abnormalities appear to be reversible, they could trigger subsequent cellular alterations in the brain and accelerate neurodegeneration-the most dangerous complication of chronic amphetamine-like drug abuse.
Figures








References
-
- Alberts DS, Sonsalla PK. Methamphetamine-induced hyperthermia and dopaminergic neurotoxicity in mice: Pharmacological profile of protective and nonprotective agents. J Pharmacol Exp Ther. 1995;275:1104–1114. - PubMed
-
- Ali SF, Newport GD, Slikker W. Methamphetamine-induced dopaminergic toxicity in mice: Role of environmental temperature and pharmacological agents. Ann NY Acad Sci. 1996;801:187–198. - PubMed
-
- Bechtold DA, Brown IR. Induction of Hsp27 and Hsp32 stress proteins and vimentin in glial cells of the rat hippocampus following hyperthermia. Neurochem Res. 2003;28:1163–1173. - PubMed
-
- Bekay L, Lee JC, Lee GC, Peng GR. Experimental cerebral concussion: An electron microscopic study. J Neurosurg. 1977;47:525–531. - PubMed
-
- Blezer E. Techniques for measuring the blood-brain barrier integrity. In: de Vries E, Prat A, editors. the Blood-Brain Barrier and Its Microenvironment. New York: Taylor & Francis; 2005. pp. 441–456.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

