Reentry in an accessory atrioventricular pathway as a trigger for atrial fibrillation initiation in manifest Wolff-Parkinson-White syndrome: a matter of reflection?
- PMID: 18774096
- PMCID: PMC2597634
- DOI: 10.1016/j.hrthm.2008.05.028
Reentry in an accessory atrioventricular pathway as a trigger for atrial fibrillation initiation in manifest Wolff-Parkinson-White syndrome: a matter of reflection?
Abstract
Background: Patients with an accessory pathway (AP) have an increased propensity to develop atrial fibrillation (AF), but the mechanism is unknown.
Objective: The purpose of this study was to identify crucial risk factors and to test the hypothesis that reflection and/or microreentry of atrial impulses propagating into the AP triggers AF.
Methods: Five hundred thirty-four patients successfully treated with radiofrequency ablation of AP at two university hospitals were evaluated. Patients were separated into those with concealed vs those with manifest AP in terms of their propensity to develop AF. To investigate AF triggering mechanisms, linear and branched two-dimensional models of atrium-to-ventricle propagation across a heterogeneous 1 x 6 AP using human ionic kinetics were simulated.
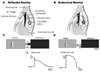
Results: A history of AF was twice as common in patients with manifest AP vs concealed AP irrespective of AP location. AF was more likely to occur in older males and in patients with larger atria. There was no correlation between AF history and AP refractory measures. However, the electrophysiologic properties of APs seemed to fulfill the prerequisites for reflection and/or microreentry of atrially initiated impulses. In the linear AP model, repetitive atrial stimulation resulted in progressively larger delay of atrium-to-ventricle propagation across the passive segment. Eventually, sufficient time for repolarization of the atrial segment allowed for reflection of an impulse that activated the entire atrium and by wavefront-wavetail interaction with a new atrial stimulus AF reentry was initiated. Simulations using the branched model showed that microreentry at the ventricular insertion of the AP could also initiate AF via retrograde atrial activation as a result of unidirectional block at the AP-ventricle junction.
Conclusion: Propensity for AF in patients with an AP is strongly related to preexcitation, larger atria, male gender, and older age. Reflection and microreentry at the AP may be important for AF initiation in patients with manifest (preexcited) Wolff-Parkinson-White syndrome. Similar mechanisms also may trigger AF in patients without an AP.
Conflict of interest statement
There are no conflicts of interest related to this work.
Figures




Comment in
-
Latent atrial fibrillation triggers originating in accessory pathways.Heart Rhythm. 2008 Sep;5(9):1248-9. doi: 10.1016/j.hrthm.2008.06.015. Epub 2008 Jun 27. Heart Rhythm. 2008. PMID: 18774097 Free PMC article. No abstract available.
References
-
- Oddsson H, Edvardsson N, Walfridsson H. Episodes of atrial fibrillation and atrial vulnerability after successful radiofrequency catheter ablation in patients with Wolff-Parkinson-White. Europace. 2002;4:201–206. - PubMed
-
- Dagres N, Clague JR, Kottkamp H, et al. Impact of radiofrequency catheter ablation of accessory pathways on the frequency of atrial fibrillation during long-term follow-up. High recurrence rate of atrial fibrillation in patients older than 50 years of age. Eur Heart J. 2001;22:423–427. - PubMed
-
- Della Bella P, Brugada P, Talajic M, et al. Atrial fibrillation in patients with an accessory pathway: importance of the conduction properties of the accessory pathway. J Am Coll Cardiol. 1991;17:1352–1356. - PubMed
-
- Antzelevitch C, Jalife J, Moe GK. Characteristics of reflection as a mechanism of reentrant arrhythmias and its relation to parasystole. Circulation. 1980;61:182–191. - PubMed
-
- Jalife J, Moe GK. Excitation, conduction, and reflection of impulses in isolated bovine and canine cardiac Purkinje fibers. Circ Res. 1981;49:233–247. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

