M2@C79N (M = Y, Tb): isolation and characterization of stable endohedral metallofullerenes exhibiting M-M bonding interactions inside aza[80]fullerene cages
- PMID: 18774804
- PMCID: PMC2696175
- DOI: 10.1021/ja802417d
M2@C79N (M = Y, Tb): isolation and characterization of stable endohedral metallofullerenes exhibiting M-M bonding interactions inside aza[80]fullerene cages
Abstract
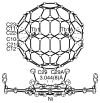
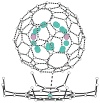
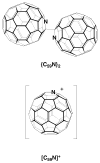
Y2@C79N and Tb2@C79N have been prepared by conducting the Kratschmer-Huffman electric-arc process under 20 Torr of N2 and 280 Torr of He with metal oxide-doped graphite rods. These new heterofullerenes were separated from the resulting mixture of empty cage fullerenes and endohedral fullerenes by chemical separation and a two-stage chromatographic process. Crystallographic data for Tb2@C79N x Ni(OEP) x 2 C6H6 demonstrate the presence of an 80-atom cage with idealized I(h) symmetry and two, widely separated Tb atoms inside with a Tb-Tb separation of 3.9020(10) A for the major terbium sites. The EPR spectrum of the odd-electron Y2@C79N indicates that the spin density largely resides on the two equivalent yttrium ions. Computational studies on Y2@C79N suggest that the nitrogen atom resides at a 665 ring junction in the equator on the fullerene cage and that the unpaired electron is localized in a bonding orbital between the two yttrium ions of this stable radical. Thus, the Tb-Tb bond length of the single-electron bond is an exceedingly long metal-metal bond.
Figures



Similar articles
-
Isolation and structural characterization of a family of endohedral fullerenes including the large, chiral cage fullerenes Tb(3)N@C(88) and Tb(3)N@C(86) as well as the I(h) and D(5)(h) isomers of Tb(3)N@C(80).J Am Chem Soc. 2007 Feb 21;129(7):2035-43. doi: 10.1021/ja066437+. Epub 2007 Jan 26. J Am Chem Soc. 2007. PMID: 17256857
-
Single-Electron Lanthanide-Lanthanide Bonds Inside Fullerenes toward Robust Redox-Active Molecular Magnets.Acc Chem Res. 2019 Oct 15;52(10):2981-2993. doi: 10.1021/acs.accounts.9b00373. Epub 2019 Oct 1. Acc Chem Res. 2019. PMID: 31571482 Free PMC article.
-
Construction of a short metallofullerene-peapod with a spin probe.Chem Commun (Camb). 2019 Sep 24;55(77):11511-11514. doi: 10.1039/c9cc05220h. Chem Commun (Camb). 2019. PMID: 31490471
-
Current status and future developments of endohedral metallofullerenes.Chem Soc Rev. 2012 Dec 7;41(23):7723-60. doi: 10.1039/c2cs35214a. Chem Soc Rev. 2012. PMID: 22907208 Review.
-
Gd@C82 Fullerenol.2008 Oct 31 [updated 2008 Dec 1]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2008 Oct 31 [updated 2008 Dec 1]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641737 Free Books & Documents. Review.
Cited by
-
Mononuclear Clusterfullerene Single-Molecule Magnet Containing Strained Fused-Pentagons Stabilized by a Nearly Linear Metal Cyanide Cluster.Angew Chem Int Ed Engl. 2017 Feb 6;56(7):1830-1834. doi: 10.1002/anie.201611345. Epub 2017 Jan 12. Angew Chem Int Ed Engl. 2017. PMID: 28079303 Free PMC article.
-
DFT Calculations of Structure and IR Spectra of M@C60 and M2@C60 Endofullerenes (M=Sc and Y).Molecules. 2025 Aug 19;30(16):3421. doi: 10.3390/molecules30163421. Molecules. 2025. PMID: 40871573 Free PMC article.
-
Recent advances in single molecule magnetism of dysprosium-metallofullerenes.Dalton Trans. 2019 Feb 26;48(9):2861-2871. doi: 10.1039/c8dt05153d. Dalton Trans. 2019. PMID: 30756104 Free PMC article.
-
Giant exchange coupling and field-induced slow relaxation of magnetization in Gd2@C79N with a single-electron Gd-Gd bond.Chem Commun (Camb). 2018 Mar 15;54(23):2902-2905. doi: 10.1039/c8cc00112j. Chem Commun (Camb). 2018. PMID: 29497728 Free PMC article.
-
Single molecule magnet with an unpaired electron trapped between two lanthanide ions inside a fullerene.Nat Commun. 2017 Jul 14;8:16098. doi: 10.1038/ncomms16098. Nat Commun. 2017. PMID: 28706223 Free PMC article.
References
-
- Vostrowsky O, Hirsch A. Chem Rev. 2006;106:5191–5207. - PubMed
-
- Branz W, Billas IML, Malinowski N, Tast F, Heinebrodt M, Martin TP. J Chem Phys. 1998;109:3425.
-
- Poblet JM, Winkler K, Cancilla M, Hayashi A, Lebrilla CB, Balch AL. Chem Comm. 1999:493.
-
- Hirsch A, Nuber B. Accounts Chem Res. 1999;32:795.
-
- Hummelen JC, Knight B, Pavlovich J, Gonzalez R, Wudl F. Science. 1995;269:1554–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

