Molecular mechanisms and clinical implications of reversible protein S-glutathionylation
- PMID: 18774901
- PMCID: PMC2774718
- DOI: 10.1089/ars.2008.2089
Molecular mechanisms and clinical implications of reversible protein S-glutathionylation
Abstract
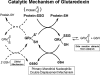
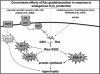
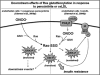
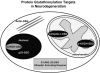
Sulfhydryl chemistry plays a vital role in normal biology and in defense of cells against oxidants, free radicals, and electrophiles. Modification of critical cysteine residues is an important mechanism of signal transduction, and perturbation of thiol-disulfide homeostasis is an important consequence of many diseases. A prevalent form of cysteine modification is reversible formation of protein mixed disulfides (protein-SSG) with glutathione (GSH). The abundance of GSH in cells and the ready conversion of sulfenic acids and S-nitroso derivatives to S-glutathione mixed disulfides suggests that reversible S-glutathionylation may be a common feature of redox signal transduction and regulation of the activities of redox sensitive thiol-proteins. The glutaredoxin enzyme has served as a focal point and important tool for evolution of this regulatory mechanism, because it is a specific and efficient catalyst of protein-SSG deglutathionylation. However, mechanisms of control of intracellular Grx activity in response to various stimuli are not well understood, and delineation of specific mechanisms and enzyme(s) involved in formation of protein-SSG intermediates requires further attention. A large number of proteins have been identified as potentially regulated by reversible S-glutathionylation, but only a few studies have documented glutathionylation-dependent changes in activity of specific proteins in a physiological context. Oxidative stress is a hallmark of many diseases which may interrupt or divert normal redox signaling and perturb protein-thiol homeostasis. Examples involving changes in S-glutathionylation of specific proteins are discussed in the context of diabetes, cardiovascular and lung diseases, cancer, and neurodegenerative diseases.
Figures






References
-
- Adachi T. Pimentel DR. Heibeck T. Hou X. Lee YJ. Jiang B. Ido Y. Cohen RA. S-glutathiolation of Ras mediates redox-sensitive signaling by angiotensin II in vascular smooth muscle cells. J Biol Chem. 2004;279:29857–29862. - PubMed
-
- Adachi T. Weisbrod RM. Pimentel DR. Ying J. Sharov VS. Schoneich C. Cohen RA. S-Glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat Med. 2004;10:1200–1207. - PubMed
-
- Aguirre V. Uchida T. Yenush L. Davis R. White MF. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307) J Biol Chem. 2000;275:9047–9054. - PubMed
-
- Aksenov MY. Markesbery WR. Changes in thiol content and expression of glutathione redox system genes in the hippocampus and cerebellum in Alzheimer's disease. Neurosci Lett. 2001;302:141–145. - PubMed
-
- Akterin S. Cowburn RF. Miranda–Vizuete A. Jimenez A. Bogdanovic N. Winblad B. Cedazo–Minguez A. Involvement of glutaredoxin-1 and thioredoxin-1 in betaamyloid toxicity and Alzheimer's disease. Cell Death Differ. 2006;13:1454–1465. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

