The meter of metabolism
- PMID: 18775307
- PMCID: PMC3760165
- DOI: 10.1016/j.cell.2008.08.022
The meter of metabolism
Abstract
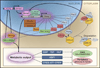
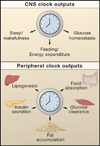
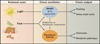
The circadian system orchestrates the temporal organization of many aspects of physiology, including metabolism, in synchrony with the 24 hr rotation of the Earth. Like the metabolic system, the circadian system is a complex feedback network that involves interactions between the central nervous system and peripheral tissues. Emerging evidence suggests that circadian regulation is intimately linked to metabolic homeostasis and that dysregulation of circadian rhythms can contribute to disease. Conversely, metabolic signals also feed back into the circadian system, modulating circadian gene expression and behavior. Here, we review the relationship between the circadian and metabolic systems and the implications for cardiovascular disease, obesity, and diabetes.
Figures



References
-
- Ahima RS, Flier JS. Leptin. Annu Rev Physiol. 2000;62:413–437. - PubMed
-
- Akanmu MA, Ukponmwan OE, Katayama Y, Honda K. Neuropeptide-Y Y2-receptor agonist, PYY3-36 promotes non-rapid eye movement sleep in rat. Neurosci Res. 2006;54:165–170. - PubMed
-
- Akashi M, Takumi T. The orphan nuclear receptor RORalpha regulates circadian transcription of the mammalian core-clock Bmal1. Nat Struct Mol Biol. 2005;12:441–448. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

