Chemical and biological approaches synergize to ameliorate protein-folding diseases
- PMID: 18775310
- PMCID: PMC2650088
- DOI: 10.1016/j.cell.2008.06.037
Chemical and biological approaches synergize to ameliorate protein-folding diseases
Abstract
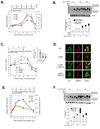
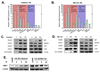
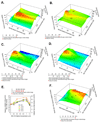
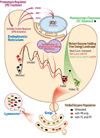
Loss-of-function diseases are often caused by a mutation in a protein traversing the secretory pathway that compromises the normal balance between protein folding, trafficking, and degradation. We demonstrate that the innate cellular protein homeostasis, or proteostasis, capacity can be enhanced to fold mutated enzymes that would otherwise misfold and be degraded, using small molecule proteostasis regulators. Two proteostasis regulators are reported that alter the composition of the proteostasis network in the endoplasmic reticulum through the unfolded protein response, increasing the mutant folded protein concentration that can engage the trafficking machinery, restoring function to two nonhomologous mutant enzymes associated with distinct lysosomal storage diseases. Coapplication of a pharmacologic chaperone and a proteostasis regulator exhibits synergy because of the former's ability to further increase the concentration of trafficking-competent mutant folded enzymes. It may be possible to ameliorate loss-of-function diseases by using proteostasis regulators alone or in combination with a pharmacologic chaperone.
Figures



References
-
- Albanese V, Yam AY-W, Baughman J, Parnot C, Frydman J. Systems analyses reveal two chaperone networks with distinct functions in eukaryotic cells. Cell. 2006;124:75–88. - PubMed
-
- Awasthi N, Wagner BJ. Upregulation of heat shock protein expression by proteasome inhibition: An antiapoptotic mechanism in the lens. Invest Ophthalmol Vis Sci. 2005;46:2082–2091. - PubMed
-
- Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–919. - PubMed
-
- Beutler E. Lysosomal storage diseases: natural history and ethical and economic aspects. Mol Genet Metab. 2006;88:208–215. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

