Lymphocyte-specific compensation for XLF/cernunnos end-joining functions in V(D)J recombination
- PMID: 18775323
- PMCID: PMC2630261
- DOI: 10.1016/j.molcel.2008.07.017
Lymphocyte-specific compensation for XLF/cernunnos end-joining functions in V(D)J recombination
Abstract
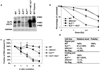
Mutations in XLF/Cernunnos (XLF) cause lymphocytopenia in humans, and various studies suggest an XLF role in classical nonhomologous end joining (C-NHEJ). We now find that XLF-deficient mouse embryonic fibroblasts are ionizing radiation (IR) sensitive and severely impaired for ability to support V(D)J recombination. Yet mature lymphocyte numbers in XLF-deficient mice are only modestly decreased. Moreover, XLF-deficient pro-B lines, while IR-sensitive, perform V(D)J recombination at nearly wild-type levels. Correspondingly, XLF/p53-double-deficient mice are not markedly prone to the pro-B lymphomas that occur in previously characterized C-NHEJ/p53-deficient mice; however, like other C-NHEJ/p53-deficient mice, they still develop medulloblastomas. Despite nearly normal V(D)J recombination in developing B cells, XLF-deficient mature B cells are moderately defective for immunoglobulin heavy-chain class switch recombination. Together, our results implicate XLF as a C-NHEJ factor but also indicate that developing mouse lymphocytes harbor cell-type-specific factors/pathways that compensate for the absence of XLF function during V(D)J recombination.
Figures





References
-
- Ahnesorg P, Smith P, Jackson SP. XLF interacts with the XRCC4-DNA ligase IV complex to promote DNA nonhomologous end-joining. Cell. 2006;124:301–313. - PubMed
-
- Andres SN, Modesti M, Tsai CJ, Chu G, Junop MS. Crystal Structure of Human XLF: A Twist in Nonhomologous DNA End-Joining. Mol. Cell. 2007;28:1093–1101. - PubMed
-
- Bassing CH, Suh H, Ferguson DO, Chua KF, Manis J, Eckersdorff M, Gleason M, Bronson R, Lee C, Alt FW. Histone H2AX: a dosage-dependent suppressor of oncogenic translocations and tumors. Cell. 2003;114:359–370. - PubMed
-
- Bredemeyer AL, Sharma GG, Huang CY, Helmink BA, Walker LM, Khor KC, Nuskey B, Sullivan KE, Pandita TK, Bassing CH, Sleckman BP. ATM stabilizes DNA double-strand-break complexes during V(D)J recombination. Nature. 2006;442:466–470. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

