Purification and characterization of flavin-containing monooxygenase isoform 3 from rat kidney microsomes
- PMID: 18775983
- PMCID: PMC2585157
- DOI: 10.1124/dmd.108.021436
Purification and characterization of flavin-containing monooxygenase isoform 3 from rat kidney microsomes
Abstract
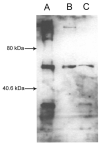
Rats are a common animal model for metabolism and toxicity studies. Previously, the enzymatic properties of rat flavin-containing monooxygenase (FMO) 1 purified from hepatic and renal microsomes and that of FMO3 purified from hepatic microsomes were characterized. This study investigated the physical, immunological, and enzymatic properties of FMO3 purified from male rat kidney microsomes and compared the results with those obtained with isolated rat liver FMO3. Renal FMO3 was purified via affinity columns based on the elution of L-methionine (Met) S-oxidase activity and reactivity of the eluted proteins with human FMO3 antibody. In general, Met S-oxidase-specific activity was increased 100-fold through the purification steps. The resulting protein had similar mobility (approximately 56 kDa) as isolated rat liver FMO3 and cDNA-expressed human FMO3 by SDS-polyacrylamide gel electrophoresis. When the isolated kidney protein band was subjected to trypsin digestion and matrix-assisted laser desorption ionization/time of flight mass spectral analysis, 34% of the sequence of rat FMO3 was detected. The apparent K(m) and V(max) values for rat kidney FMO3 were determined using the known FMO substrates Met, seleno-L-methionine, S-allyl-L-cysteine (SAC), and methimazole (N-methyl-2-mercaptoimidazole). The stereoselectivity of the reactions with Met and SAC were also examined using high-performance liquid chromatography. The obtained kinetic and stereoselectivity results were similar to those we obtained in the present study, or those previously reported, for rat liver FMO3. Taken together, the results demonstrate many similar properties between rat hepatic and renal FMO3 forms and suggest that renal FMO3 may play an important role in kidney metabolism of xenobiotics containing sulfur and selenium atoms.
Figures


Similar articles
-
Characterization of the methionine S-oxidase activity of rat liver and kidney microsomes: immunochemical and kinetic evidence for FMO3 being the major catalyst.Arch Biochem Biophys. 1996 Sep 1;333(1):109-16. doi: 10.1006/abbi.1996.0370. Arch Biochem Biophys. 1996. PMID: 8806760
-
Methionine S-oxidation in human and rabbit liver microsomes: evidence for a high-affinity methionine S-oxidase activity that is distinct from flavin-containing monooxygenase 3.Arch Biochem Biophys. 1999 Jul 15;367(2):322-32. doi: 10.1006/abbi.1999.1247. Arch Biochem Biophys. 1999. PMID: 10395751
-
Species and sex differences in expression of flavin-containing monooxygenase form 3 in liver and kidney microsomes.Drug Metab Dispos. 1999 Jan;27(1):46-52. Drug Metab Dispos. 1999. PMID: 9884308
-
Potential role of the flavin-containing monooxygenases in the metabolism of endogenous compounds.Chem Biol Interact. 1995 Apr 28;96(1):47-55. doi: 10.1016/0009-2797(94)03582-s. Chem Biol Interact. 1995. PMID: 7720104 Review.
-
Role of hepatic flavin-containing monooxygenase 3 in drug and chemical metabolism in adult humans.Chem Biol Interact. 1995 Apr 28;96(1):33-46. doi: 10.1016/0009-2797(94)03581-r. Chem Biol Interact. 1995. PMID: 7720103 Review.
Cited by
-
Isoform distinct time-, dose-, and castration-dependent alterations in flavin-containing monooxygenase expression in mouse liver after 2,3,7,8-tetrachlorodibenzo-p-dioxin treatment.Biochem Pharmacol. 2010 May 1;79(9):1345-51. doi: 10.1016/j.bcp.2009.12.020. Epub 2009 Dec 28. Biochem Pharmacol. 2010. PMID: 20036217 Free PMC article.
-
Trichloroethylene biotransformation and its role in mutagenicity, carcinogenicity and target organ toxicity.Mutat Res Rev Mutat Res. 2014 Oct-Dec;762:22-36. doi: 10.1016/j.mrrev.2014.04.003. Mutat Res Rev Mutat Res. 2014. PMID: 25484616 Free PMC article. Review.
-
Regulation of protein function by reversible methionine oxidation and the role of selenoprotein MsrB1.Antioxid Redox Signal. 2015 Oct 1;23(10):814-22. doi: 10.1089/ars.2015.6385. Epub 2015 Jul 16. Antioxid Redox Signal. 2015. PMID: 26181576 Free PMC article. Review.
-
Reduction of L-methionine selenoxide to seleno-L-methionine by endogenous thiols, ascorbic acid, or methimazole.Biochem Pharmacol. 2009 Jan 1;77(1):134-40. doi: 10.1016/j.bcp.2008.09.022. Epub 2008 Sep 27. Biochem Pharmacol. 2009. PMID: 18930712 Free PMC article.
References
-
- Borbás T, Benkő B, Dalmadi B, Szabó I, Tihanyi K. Insulin in flavin-containing monooxygenase regulation. Flavin-containing monooxygenase and cytochrome P450 activities in experimental diabetes. Eur J Pharm Sci. 2006;28:51–58. - PubMed
-
- Bosy-Westphal A, Ruschmeyer M, Czech N, Oehler G, Hinrichsen H, Plauth M, Lotterer E, Fleig W, Müller MJ. Determinants of hyperhomocysteinemia in patients with chronic liver disease and after orthotopic liver transplantation. Am J Clin Nutr. 2003;77:1269–1277. - PubMed
-
- Burnett VL, Lawton MP, Philpot RM. Cloning and sequencing of flavin-containing monooxygenases FMO3 and FMO4 from rabbit and characterization of FMO3*. J Biol Chem. 1994;269:14314–14322. - PubMed
-
- Cashman JR, Zhang J. Human flavin-containing monooxygenases. Annu Rev Pharmacol Toxicol. 2006;46:65–100. - PubMed
-
- Dixit A, Roche TE. Spectrophotometric assay of the flavin-containing monooxygenase and changes in its activity in female mouse liver with nutritional and dinurnal conditions. Arch Biochem Biophys. 1984;233:50–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

