Endoglin promotes transforming growth factor beta-mediated Smad 1/5/8 signaling and inhibits endothelial cell migration through its association with GIPC
- PMID: 18775991
- PMCID: PMC2583306
- DOI: 10.1074/jbc.M803059200
Endoglin promotes transforming growth factor beta-mediated Smad 1/5/8 signaling and inhibits endothelial cell migration through its association with GIPC
Abstract
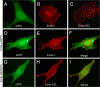
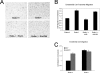
Transforming growth factor beta (TGF-beta) signals through two distinct pathways to regulate endothelial cell proliferation, migration, and angiogenesis, the ALK-1/Smad 1/5/8 and ALK-5/Smad2/3 pathways. Endoglin is a co-receptor predominantly expressed in endothelial cells that participates in TGFbeta-mediated signaling with ALK-1 and ALK-5 and regulates critical aspects of cellular and biological responses. The embryonic lethal phenotype of knock-out mice because of defects in angiogenesis and disease-causing mutations resulting in human vascular diseases both support essential roles for endoglin, ALK-1, and ALK-5 in the vasculature. However, the mechanism by which endoglin mediates TGF-beta signaling through ALK-1 and ALK-5 has remained elusive. Here we describe a novel interaction between endoglin and GIPC, a scaffolding protein known to regulate cell surface receptor expression and trafficking. Co-immunoprecipitation and immunofluorescence confocal studies both demonstrate a specific interaction between endoglin and GIPC in endothelial cells, mediated by a class I PDZ binding motif in the cytoplasmic domain of endoglin. Subcellular distribution studies demonstrate that endoglin recruits GIPC to the plasma membrane and co-localizes with GIPC in a TGFbeta-independent manner, with GIPC-promoting cell surface retention of endoglin. Endoglin specifically enhanced TGF-beta1-induced phosphorylation of Smad 1/5/8, increased a Smad 1/5/8 responsive promoter, and inhibited endothelial cell migration in a manner dependent on the ability of endoglin to interact with GIPC. These studies define a novel mechanism for the regulation of endoglin signaling and function in endothelial cells and demonstrate a new role for GIPC in TGF-beta signaling.
Figures




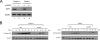
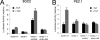

References
-
- Massague, J. (1998) Annu. Rev. Biochem. 67, 753-791 - PubMed
-
- Cheifetz, S., Andres, J. L. and Massague, J. (1988) J. Biol. Chem. 263 16984-16991 - PubMed
-
- Cheifetz, S., Bellón, T., Calés, C., Vera, S., Bernabeu, C., Massagué, J., and Letarte, M. (1992) J. Biol. Chem. 267 19027-19030 - PubMed
-
- Barbara, N. P., Wrana, J. L., and Letarte, M. (1999) J. Biol. Chem. 274 584-594 - PubMed
-
- Ten Dijke, P., Goumans, M. J., Itoh, F., and Itoh, S. (2002) J. Cell. Physiol. 191 1-16 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

