A functional phenylacetic acid catabolic pathway is required for full pathogenicity of Burkholderia cenocepacia in the Caenorhabditis elegans host model
- PMID: 18776009
- PMCID: PMC2580687
- DOI: 10.1128/JB.00481-08
A functional phenylacetic acid catabolic pathway is required for full pathogenicity of Burkholderia cenocepacia in the Caenorhabditis elegans host model
Abstract
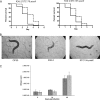
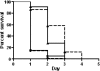
Burkholderia cenocepacia is a member of the Burkholderia cepacia complex, a group of metabolically versatile bacteria that have emerged as opportunistic pathogens in cystic fibrosis and immunocompromised patients. Previously a screen of transposon mutants in a rat pulmonary infection model identified an attenuated mutant with an insertion in paaE, a gene related to the phenylacetic acid (PA) catabolic pathway. In this study, we characterized gene clusters involved in the PA degradation pathway of B. cenocepacia K56-2 in relation to its pathogenicity in the Caenorhabditis elegans model of infection. We demonstrated that targeted-insertion mutagenesis of paaA and paaE, which encode part of the putative PA-coenzyme A (CoA) ring hydroxylation system, paaZ, coding for a putative ring opening enzyme, and paaF, encoding part of the putative beta-oxidation system, severely reduces growth on PA as a sole carbon source. paaA and paaE insertional mutants were attenuated for virulence, and expression of paaE in trans restored pathogenicity of the paaE mutant to wild-type levels. Interruption of paaZ and paaF slightly increased virulence. Using gene interference by ingested double-stranded RNA, we showed that the attenuated phenotype of the paaA and paaE mutants is dependent on a functional p38 mitogen-activated protein kinase pathway in C. elegans. Taken together, our results demonstrate that B. cenocepacia possesses a functional PA degradation pathway and that the putative PA-CoA ring hydroxylation system is required for full pathogenicity in C. elegans.
Figures




Similar articles
-
3-Hydroxyphenylacetic acid induces the Burkholderia cenocepacia phenylacetic acid degradation pathway - toward understanding the contribution of aromatic catabolism to pathogenesis.Front Cell Infect Microbiol. 2011 Dec 14;1:14. doi: 10.3389/fcimb.2011.00014. eCollection 2011. Front Cell Infect Microbiol. 2011. PMID: 22919580 Free PMC article.
-
Regulation of phenylacetic acid degradation genes of Burkholderia cenocepacia K56-2.BMC Microbiol. 2009 Oct 18;9:222. doi: 10.1186/1471-2180-9-222. BMC Microbiol. 2009. PMID: 19835630 Free PMC article.
-
Phenylalanine induces Burkholderia cenocepacia phenylacetic acid catabolism through degradation to phenylacetyl-CoA in synthetic cystic fibrosis sputum medium.Microb Pathog. 2011 Sep;51(3):186-93. doi: 10.1016/j.micpath.2011.04.002. Epub 2011 Apr 13. Microb Pathog. 2011. PMID: 21511027
-
The type 2 secretion Pseudopilin, gspJ, is required for multihost pathogenicity of Burkholderia cenocepacia AU1054.Infect Immun. 2010 Oct;78(10):4110-21. doi: 10.1128/IAI.00558-10. Epub 2010 Jul 26. Infect Immun. 2010. PMID: 20660607 Free PMC article.
-
Progress in structural and functional study of the bacterial phenylacetic acid catabolic pathway, its role in pathogenicity and antibiotic resistance.Front Microbiol. 2022 Sep 8;13:964019. doi: 10.3389/fmicb.2022.964019. eCollection 2022. Front Microbiol. 2022. PMID: 36160191 Free PMC article. Review.
Cited by
-
Transcriptomic analysis reveals the regulatory role of quorum sensing in the Acinetobacter baumannii ATCC 19606 via RNA-seq.BMC Microbiol. 2022 Aug 16;22(1):198. doi: 10.1186/s12866-022-02612-z. BMC Microbiol. 2022. PMID: 35971084 Free PMC article.
-
A decade of Burkholderia cenocepacia virulence determinant research.Infect Immun. 2010 Oct;78(10):4088-100. doi: 10.1128/IAI.00212-10. Epub 2010 Jul 19. Infect Immun. 2010. PMID: 20643851 Free PMC article. Review.
-
Identification of specific and universal virulence factors in Burkholderia cenocepacia strains by using multiple infection hosts.Infect Immun. 2009 Sep;77(9):4102-10. doi: 10.1128/IAI.00398-09. Epub 2009 Jun 15. Infect Immun. 2009. PMID: 19528212 Free PMC article.
-
Identification of determinants that allow maintenance of high-level fluoroquinolone resistance in Acinetobacter baumannii.mBio. 2025 Jan 8;16(1):e0322124. doi: 10.1128/mbio.03221-24. Epub 2024 Nov 26. mBio. 2025. PMID: 39589129 Free PMC article.
-
Transcriptomic investigations of polymyxins and colistin/sulbactam combination against carbapenem-resistant Acinetobacter baumannii.Comput Struct Biotechnol J. 2024 May 31;23:2595-2605. doi: 10.1016/j.csbj.2024.05.043. eCollection 2024 Dec. Comput Struct Biotechnol J. 2024. PMID: 39006922 Free PMC article.
References
-
- Aballay, A., and F. M. Ausubel. 2002. Caenorhabditis elegans as a host for the study of host-pathogen interactions. Curr. Opin. Microbiol. 597-101. - PubMed
-
- Avery, L., and H. R. Horvitz. 1990. Effects of starvation and neuroactive drugs on feeding in Caenorhabditis elegans. J. Exp. Zool. 253263-270. - PubMed
-
- Cardona, S. T., and M. A. Valvano. 2005. An expression vector containing a rhamnose-inducible promoter provides tightly regulated gene expression in Burkholderia cenocepacia. Plasmid 54219-228. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources

