The bile response repressor BreR regulates expression of the Vibrio cholerae breAB efflux system operon
- PMID: 18776020
- PMCID: PMC2576662
- DOI: 10.1128/JB.00584-08
The bile response repressor BreR regulates expression of the Vibrio cholerae breAB efflux system operon
Abstract
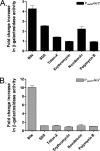
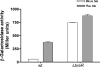
Enteric pathogens have developed several resistance mechanisms to survive the antimicrobial action of bile. We investigated the transcriptional profile of Vibrio cholerae O1 El Tor strain C6706 under virulence gene-inducing conditions in the presence and absence of bile. Microarray analysis revealed that the expression of 119 genes was affected by bile. The mRNA levels of genes encoding proteins involved in transport were increased in the presence of bile, whereas the mRNA levels of genes encoding proteins involved in pathogenesis and chemotaxis were decreased. This study identified genes encoding transcriptional regulators from the TetR family (vexR and breR) and multidrug efflux pumps from the resistance-nodulation-cell division superfamily (vexB and vexD [herein renamed breB]) that were induced in response to bile. Further analysis regarding vexAB and breAB expression in the presence of various antimicrobial compounds established that vexAB was induced in the presence of bile, sodium dodecyl sulfate, or novobiocin and that the induction of breAB was specific to bile. BreR is a direct repressor of the breAB promoter and is able to regulate its own expression, as demonstrated by transcriptional and electrophoretic mobility shift assays (EMSA). The expression of breR and breAB is induced in the presence of the bile salts cholate, deoxycholate, and chenodeoxycholate, and EMSA showed that deoxycholate is able to abolish the formation of BreR-P(breR) complexes. We propose that deoxycholate is able to interact with BreR and induce a conformational change that interferes with the DNA binding ability of BreR, resulting in breAB and breR expression. These results provide new insight into a transcriptional regulator and a transport system that likely play essential roles in the ability of V. cholerae to resist the action of bile in the host.
Figures








References
-
- Begley, M., C. G. Gahan, and C. Hill. 2005. The interaction between bacteria and bile. FEMS Microbiol. Rev. 29625-651. - PubMed
-
- Bina, J. E., D. Provenzano, C. Wang, X. R. Bina, and J. J. Mekalanos. 2006. Characterization of the Vibrio cholerae vexAB and vexCD efflux systems. Arch. Microbiol. 186171-181. - PubMed
-
- Blanco, C., M. Mata-Gilsinger, and P. Ritzenthaler. 1985. The use of gene fusions to study the expression of uidR, a negative regulatory gene of Escherichia coli K-12. Gene 36159-167. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

