Effects of cholecystokinin-58 on type 1 cholecystokinin receptor function and regulation
- PMID: 18776046
- PMCID: PMC2536789
- DOI: 10.1152/ajpgi.90390.2008
Effects of cholecystokinin-58 on type 1 cholecystokinin receptor function and regulation
Abstract
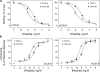
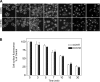
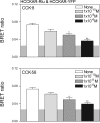
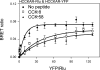
Cholecystokinin, like many peptide hormones, is present as multiple molecular forms. CCK-58 has been identified as the dominant form in the circulation, whereas most of the studies of CCK-receptor interactions have been performed with CCK-8. Despite both sharing the pharmacophoric region of CCK, representing its carboxy terminal heptapeptide amide, studies in vivo have demonstrated biological diversity of action of the two peptides, with CCK-58, but not CCK-8, stimulating pancreatic fluid secretion and lengthening the interval between meals. Here, we have directly studied the ability of these two CCK peptides to bind to the type 1 CCK receptor and to stimulate it to elicit an intracellular calcium response. The calcium response relative to receptor occupation was identical for CCK-58 and CCK-8, with the longer peptide binding with approximately fivefold lower affinity. We also examined the ability of the two peptides to elicit receptor internalization using morphological techniques and to disrupt the constitutive oligomerization of the CCK receptor using receptor bioluminescence resonance energy transfer. Here, both full agonist peptides had similar effects on these regulatory processes. These data suggest that both molecular forms of CCK act at the CCK1 receptor quite similarly and elicit similar regulatory processes for that receptor, suggesting that the differences in biological activity observed in vivo most likely reflect differences in the clearance and/or metabolism of these long and short forms of CCK peptides.
Figures
Similar articles
-
Identification of two amino acids of the human cholecystokinin-A receptor that interact with the N-terminal moiety of cholecystokinin.J Biol Chem. 1997 Jan 31;272(5):2920-6. doi: 10.1074/jbc.272.5.2920. J Biol Chem. 1997. PMID: 9006937
-
Full and partial agonist activity of C-terminal cholecystokinin peptides at the cloned human CCK-A receptor expressed in Chinese hamster ovary cells.Peptides. 1997;18(6):865-8. doi: 10.1016/s0196-9781(97)00012-0. Peptides. 1997. PMID: 9285936
-
Visualization of G protein-coupled receptor trafficking with the aid of the green fluorescent protein. Endocytosis and recycling of cholecystokinin receptor type A.J Biol Chem. 1997 Jun 6;272(23):14817-24. doi: 10.1074/jbc.272.23.14817. J Biol Chem. 1997. PMID: 9169450
-
Natural and synthetic CCK-58. Novel reagents for studying cholecystokinin physiology.Ann N Y Acad Sci. 1994 Mar 23;713:11-21. doi: 10.1111/j.1749-6632.1994.tb44047.x. Ann N Y Acad Sci. 1994. PMID: 7514372 Review.
-
Therapeutic potential for novel drugs targeting the type 1 cholecystokinin receptor.Br J Pharmacol. 2010 Mar;159(5):1009-21. doi: 10.1111/j.1476-5381.2009.00489.x. Epub 2009 Nov 18. Br J Pharmacol. 2010. PMID: 19922535 Free PMC article. Review.
Cited by
-
CCK-58 elicits both satiety and satiation in rats while CCK-8 elicits only satiation.Peptides. 2014 Apr;54:71-80. doi: 10.1016/j.peptides.2014.01.008. Epub 2014 Jan 24. Peptides. 2014. PMID: 24468546 Free PMC article.
-
Regulation of acinar cell function in the pancreas.Curr Opin Gastroenterol. 2010 Sep;26(5):478-83. doi: 10.1097/MOG.0b013e32833d11c6. Curr Opin Gastroenterol. 2010. PMID: 20625287 Free PMC article. Review.
-
Discovery of a Positive Allosteric Modulator of Cholecystokinin Action at CCK1R in Normal and Elevated Cholesterol.Front Endocrinol (Lausanne). 2021 Dec 7;12:789957. doi: 10.3389/fendo.2021.789957. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34950108 Free PMC article.
-
Systemic cholecystokinin amplifies vago-vagal reflex responses recorded in vagal motor neurones.J Physiol. 2012 Feb 1;590(3):631-46. doi: 10.1113/jphysiol.2011.224477. Epub 2011 Dec 12. J Physiol. 2012. PMID: 22155934 Free PMC article.
-
CCK-8 and CCK-58 differ in their effects on nocturnal solid meal pattern in undisturbed rats.Am J Physiol Regul Integr Comp Physiol. 2012 Oct 15;303(8):R850-60. doi: 10.1152/ajpregu.00365.2011. Epub 2012 Aug 8. Am J Physiol Regul Integr Comp Physiol. 2012. PMID: 22874423 Free PMC article.
References
-
- Angers S, Salahpour A, Bouvier M. Dimerization: an emerging concept for G protein-coupled receptor ontogeny and function. Annu Rev Pharmacol Toxicol 42: 409–435, 2002. - PubMed
-
- Burton-Freeman B, Gietzen DW, Schneeman BO. Cholecystokinin and serotonin receptors in the regulation of fat-induced satiety in rats. Am J Physiol Regul Integr Comp Physiol 276: R429–R434, 1999. - PubMed
-
- Cheng ZJ, Harikumar KG, Holicky EL, Miller LJ. Heterodimerization of type A and B cholecystokinin receptors enhance signaling and promote cell growth. J Biol Chem 278: 52972–52979, 2003. - PubMed
-
- Cheng ZJ, Miller LJ. Agonist-dependent dissociation of oligomeric complexes of G protein-coupled cholecystokinin receptors demonstrated in living cells using bioluminescence resonance energy transfer. J Biol Chem 276: 48040–48047, 2001. - PubMed
-
- Dong M, Ding XQ, Thomas SE, Gao F, Lam PC, Abagyan R, Miller LJ. Role of lysine187 within the second extracellular loop of the type A cholecystokinin receptor in agonist-induced activation. Use of complementary charge-reversal mutagenesis to define a functionally important interdomain interaction. Biochemistry 46: 4522–4531, 2007. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

