Effects of cholecystokinin-58 on type 1 cholecystokinin receptor function and regulation
- PMID: 18776046
- PMCID: PMC2536789
- DOI: 10.1152/ajpgi.90390.2008
Effects of cholecystokinin-58 on type 1 cholecystokinin receptor function and regulation
Abstract
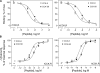
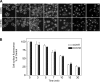
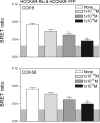
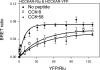
Cholecystokinin, like many peptide hormones, is present as multiple molecular forms. CCK-58 has been identified as the dominant form in the circulation, whereas most of the studies of CCK-receptor interactions have been performed with CCK-8. Despite both sharing the pharmacophoric region of CCK, representing its carboxy terminal heptapeptide amide, studies in vivo have demonstrated biological diversity of action of the two peptides, with CCK-58, but not CCK-8, stimulating pancreatic fluid secretion and lengthening the interval between meals. Here, we have directly studied the ability of these two CCK peptides to bind to the type 1 CCK receptor and to stimulate it to elicit an intracellular calcium response. The calcium response relative to receptor occupation was identical for CCK-58 and CCK-8, with the longer peptide binding with approximately fivefold lower affinity. We also examined the ability of the two peptides to elicit receptor internalization using morphological techniques and to disrupt the constitutive oligomerization of the CCK receptor using receptor bioluminescence resonance energy transfer. Here, both full agonist peptides had similar effects on these regulatory processes. These data suggest that both molecular forms of CCK act at the CCK1 receptor quite similarly and elicit similar regulatory processes for that receptor, suggesting that the differences in biological activity observed in vivo most likely reflect differences in the clearance and/or metabolism of these long and short forms of CCK peptides.
Figures
References
-
- Angers S, Salahpour A, Bouvier M. Dimerization: an emerging concept for G protein-coupled receptor ontogeny and function. Annu Rev Pharmacol Toxicol 42: 409–435, 2002. - PubMed
-
- Burton-Freeman B, Gietzen DW, Schneeman BO. Cholecystokinin and serotonin receptors in the regulation of fat-induced satiety in rats. Am J Physiol Regul Integr Comp Physiol 276: R429–R434, 1999. - PubMed
-
- Cheng ZJ, Harikumar KG, Holicky EL, Miller LJ. Heterodimerization of type A and B cholecystokinin receptors enhance signaling and promote cell growth. J Biol Chem 278: 52972–52979, 2003. - PubMed
-
- Cheng ZJ, Miller LJ. Agonist-dependent dissociation of oligomeric complexes of G protein-coupled cholecystokinin receptors demonstrated in living cells using bioluminescence resonance energy transfer. J Biol Chem 276: 48040–48047, 2001. - PubMed
-
- Dong M, Ding XQ, Thomas SE, Gao F, Lam PC, Abagyan R, Miller LJ. Role of lysine187 within the second extracellular loop of the type A cholecystokinin receptor in agonist-induced activation. Use of complementary charge-reversal mutagenesis to define a functionally important interdomain interaction. Biochemistry 46: 4522–4531, 2007. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

