The guanylate kinase domain of the beta-subunit of voltage-gated calcium channels suffices to modulate gating
- PMID: 18776052
- PMCID: PMC2544601
- DOI: 10.1073/pnas.0806558105
The guanylate kinase domain of the beta-subunit of voltage-gated calcium channels suffices to modulate gating
Abstract
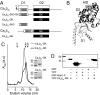
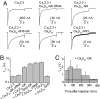
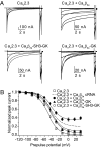
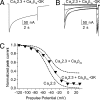
Inactivation of voltage-gated calcium channels is crucial for the spatiotemporal coordination of calcium signals and prevention of toxic calcium buildup. Only one member of the highly conserved family of calcium channel beta-subunits--Ca(V)beta--inhibits inactivation. This unique property has been attributed to short variable regions of the protein; however, here we report that this inhibition actually is conferred by a conserved guanylate kinase (GK) domain and, moreover, that this domain alone recapitulates Ca(V)beta-mediated modulation of channel activation. We expressed and refolded the GK domain of Ca(V)beta(2a), the unique variant that inhibits inactivation, and of Ca(V)beta(1b), an isoform that facilitates it. The refolded domains of both Ca(V)beta variants were found to inhibit inactivation of Ca(V)2.3 channels expressed in Xenopus laevis oocytes. These findings suggest that the GK domain endows calcium channels with a brake restraining voltage-dependent inactivation, and thus facilitation of inactivation by full-length Ca(V)beta requires additional structural determinants to antagonize the GK effect. We found that Ca(V)beta can switch the inactivation phenotype conferred to Ca(V)2.3 from slow to fast after posttranslational modifications during channel biogenesis. Our findings provide a framework within which to understand the modulation of inactivation and a new functional map of Ca(V)beta in which the GK domain regulates channel gating and the other conserved domain (Src homology 3) may couple calcium channels to other signaling pathways.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. - PubMed
-
- Pragnell M, et al. Calcium channel β-subunit binds to a conserved motif in the I-II cytoplasmic linker of the α1-subunit. Nature. 1994;368:67–70. - PubMed
-
- Harry JB, Kobrinsky E, Abernethy DR, Soldatov NM. New short splice variants of the human cardiac Cavbeta2 subunit: Redefining the major functional motifs implemented in modulation of the Cav1.2 channel. J Biol Chem. 2004;279:46367–46372. - PubMed
-
- Opatowsky Y, Chen CC, Campbell KP, Hirsch JA. Structural analysis of the voltage-dependent calcium channel beta subunit functional core and its complex with the alpha 1 interaction domain. Neuron. 2004;42:387–399. - PubMed
-
- Chen YH, et al. Structural basis of the alpha1-beta subunit interaction of voltage-gated Ca2+ channels. Nature. 2004;429:675–680. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

