Podocyte-specific deletion of dicer alters cytoskeletal dynamics and causes glomerular disease
- PMID: 18776121
- PMCID: PMC2573015
- DOI: 10.1681/ASN.2008020233
Podocyte-specific deletion of dicer alters cytoskeletal dynamics and causes glomerular disease
Abstract
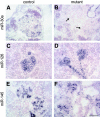
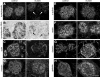
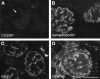
MicroRNAs (miRNAs) regulate gene expression by binding the 3' untranslated region of mRNAs. To define their role in glomerular function, miRNA biogenesis was disrupted in mouse podocytes using a conditional Dicer allele. Mutant mice developed proteinuria by 3 wk after birth and progressed rapidly to end-stage kidney disease. Podocyte pathology included effacement, vacuolization, and hypertrophy with crescent formation. Despite normal expression of WT1, podocytes underwent dedifferentiation, exemplified by cytoskeletal disruption with early transcriptional downregulation of synaptopodin. These abnormalities differed from Cd2ap(-/-) mice, indicating they were not a general consequence of glomerular disease. Glomerular labeling of ezrin, moesin, and gelsolin was altered at 3 wk, but expression of nestin and alpha-actinin was unchanged. Abnormal cell proliferation or apoptosis was not responsible for the glomerular injury. Mutant podocytes were incapable of synthesizing mature miRNA, as revealed by their loss of miR-30a. In contrast, expression of glomerular endothelial and mesangial cell miRNAs (miR-126 and miR-145, respectively) was unchanged. These findings demonstrate a critical role for miRNA in glomerular function and suggest a pathway that may participate in the pathogenesis of kidney diseases of podocyte origin. The unique architecture of podocytes may make them especially susceptible to cytoskeletal alterations initiated by aberrant miRNA dynamics.
Figures




Comment in
-
Dicer cuts the kidney.J Am Soc Nephrol. 2008 Nov;19(11):2043-6. doi: 10.1681/ASN.2008090986. Epub 2008 Oct 15. J Am Soc Nephrol. 2008. PMID: 18923053 Review. No abstract available.
References
-
- Kloosterman W, Plasterk R: The diverse functions of microRNAs in animal development and disease. Dev Cell 11: 441–450, 2006 - PubMed
-
- Bartel D, Chen C: Micromanagers of gene expression: The potentially widespread influence of metazoan microRNAs. Nat Rev Genet 5: 396–400, 2004 - PubMed
-
- Kim V: MicroRNA biogenesis: Coordinated cropping and dicing. Nat Rev Mol Cell Biol 6: 376–385, 2005 - PubMed
-
- Bernstein E, Kim S, Carmell M, Murchison E, Alcom H, Li M, Mills A, Elledge S, Anderson K, Hannon G: Dicer is essential for mouse development. Nat Genet 35: 215–217, 2003 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

