Expression of ammonia transporters, Rhbg and Rhcg, in chronic cyclosporine nephropathy in rats
- PMID: 18776723
- PMCID: PMC4321814
- DOI: 10.1159/000153245
Expression of ammonia transporters, Rhbg and Rhcg, in chronic cyclosporine nephropathy in rats
Abstract
Background/aims: Cyclosporine (CsA)-induced renal injury causes renal tubular acidosis. The current study was performed to evaluate the influence of CsA-induced renal injury on the ammonia transporter family members, Rh B-glycoprotein (Rhbg) and Rh C-glycoprotein (Rhcg).
Methods: Rats were treated daily for 1 or 4 weeks with vehicle (VH) or CsA. Induction of chronic CsA-induced nephropathy was confirmed by demonstrating impaired renal function and characteristic histopathology. Rhbg and Rhcg expression was evaluated with immunoblot, immunohistochemistry, real-time RT-PCR and electron microscopy.
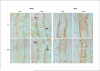
Results: CsA treatment for 4 weeks developed mild metabolic acidosis and decreased urinary ammonia excretion. Rhcg mRNA expression was unchanged in both the cortex and outer medulla, but Rhcg protein expression in the CsA group was significantly reduced in the cortex and outer medulla. There were no significant differences in Rhbg mRNA and protein expression between the CsA and VH group.
Conclusion: Long-term treatment with CsA in rats results in decreased urinary ammonia excretion accompanied by decreased expression of Rhcg; these changes are likely to mediate the CsA-induced defect in ammonium excretion in the collecting duct.
(c) 2008 S. Karger AG, Basel.
Figures




References
-
- Ling BN, Eaton DC. Cyclosporin A inhibits apical secretory K + channels in rabbit cortical collecting tubule principal cells. Kidney Int. 1993;44:974–984. - PubMed
-
- Thompson CB, June CH, Sullivan KM, Thomas ED. Association between cyclosporin neurotoxicity and hypomagnesaemia. Lancet. 1984;17:1116–1120. - PubMed
-
- Young BA, Burdmann EA, Johnson RJ, Alpers CE, Giachelli CM, Eng E, Andoh T, Bennett WM, Couser WG. Cellular proliferation and macrophage influx precede interstitial fibrosis in cyclosporine nephrotoxicity. Kidney Int. 1995;48:439–448. - PubMed
-
- Batlle DC, Gutterman C, Tarka J, Prasad R. Effect of short-term cyclosporine A administration on urinary acidification. Clin Nephrol. 1986;25(suppl 1):S62–S69. - PubMed
-
- DuBose TD, Jr, Good DW, Hamm LL, Wall SM. Ammonium transport in the kidney: new physiological concepts and their clinical implications. J Am Soc Nephrol. 1991;1:1193–1203. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

