Neural correlates of multisensory cue integration in macaque MSTd
- PMID: 18776893
- PMCID: PMC2713666
- DOI: 10.1038/nn.2191
Neural correlates of multisensory cue integration in macaque MSTd
Abstract
Human observers combine multiple sensory cues synergistically to achieve greater perceptual sensitivity, but little is known about the underlying neuronal mechanisms. We recorded the activity of neurons in the dorsal medial superior temporal (MSTd) area during a task in which trained monkeys combined visual and vestibular cues near-optimally to discriminate heading. During bimodal stimulation, MSTd neurons combined visual and vestibular inputs linearly with subadditive weights. Neurons with congruent heading preferences for visual and vestibular stimuli showed improvements in sensitivity that parallel behavioral effects. In contrast, neurons with opposite preferences showed diminished sensitivity under cue combination. Responses of congruent cells were more strongly correlated with monkeys' perceptual decisions than were responses of opposite cells, suggesting that the monkey monitored the activity of congruent cells to a greater extent during cue integration. These findings show that perceptual cue integration occurs in nonhuman primates and identify a population of neurons that may form its neural basis.
Figures


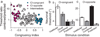



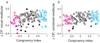
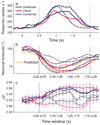
Comment in
-
Right way neurons.Nat Neurosci. 2008 Oct;11(10):1121-2. doi: 10.1038/nn1008-1121. Nat Neurosci. 2008. PMID: 18818590 No abstract available.
Similar articles
-
Binocular disparity tuning and visual-vestibular congruency of multisensory neurons in macaque parietal cortex.J Neurosci. 2011 Dec 7;31(49):17905-16. doi: 10.1523/JNEUROSCI.4032-11.2011. J Neurosci. 2011. PMID: 22159105 Free PMC article.
-
Causal links between dorsal medial superior temporal area neurons and multisensory heading perception.J Neurosci. 2012 Feb 15;32(7):2299-313. doi: 10.1523/JNEUROSCI.5154-11.2012. J Neurosci. 2012. PMID: 22396405 Free PMC article.
-
Evidence for a Causal Contribution of Macaque Vestibular, But Not Intraparietal, Cortex to Heading Perception.J Neurosci. 2016 Mar 30;36(13):3789-98. doi: 10.1523/JNEUROSCI.2485-15.2016. J Neurosci. 2016. PMID: 27030763 Free PMC article.
-
Visual and vestibular cue integration for heading perception in extrastriate visual cortex.J Physiol. 2011 Feb 15;589(Pt 4):825-33. doi: 10.1113/jphysiol.2010.194720. Epub 2010 Aug 2. J Physiol. 2011. PMID: 20679353 Free PMC article. Review.
-
Visual-vestibular cue integration for heading perception: applications of optimal cue integration theory.Eur J Neurosci. 2010 May;31(10):1721-9. doi: 10.1111/j.1460-9568.2010.07207.x. Eur J Neurosci. 2010. PMID: 20584175 Free PMC article. Review.
Cited by
-
Inferring decoding strategies from choice probabilities in the presence of correlated variability.Nat Neurosci. 2013 Feb;16(2):235-42. doi: 10.1038/nn.3309. Epub 2013 Jan 13. Nat Neurosci. 2013. PMID: 23313912
-
How vision and self-motion combine or compete during path reproduction changes with age.Sci Rep. 2016 Jul 6;6:29163. doi: 10.1038/srep29163. Sci Rep. 2016. PMID: 27381183 Free PMC article.
-
Multisensory integration in the estimation of walked distances.Exp Brain Res. 2012 May;218(4):551-65. doi: 10.1007/s00221-012-3048-1. Epub 2012 Mar 13. Exp Brain Res. 2012. PMID: 22411581
-
Combination of Interaural Level and Time Difference in Azimuthal Sound Localization in Owls.eNeuro. 2017 Dec 14;4(6):ENEURO.0238-17.2017. doi: 10.1523/ENEURO.0238-17.2017. eCollection 2017 Nov-Dec. eNeuro. 2017. PMID: 29379866 Free PMC article.
-
Supervised calibration relies on the multisensory percept.Neuron. 2013 Dec 18;80(6):1544-57. doi: 10.1016/j.neuron.2013.09.026. Epub 2013 Nov 27. Neuron. 2013. PMID: 24290205 Free PMC article.
References
-
- Jacobs RA. Optimal integration of texture and motion cues to depth. Vision Res. 1999;39:3621–3629. - PubMed
-
- Hillis JM, Watt SJ, Landy MS, Banks MS. Slant from texture and disparity cues: optimal cue combination. J Vis. 2004;4:967–992. - PubMed
-
- Knill DC, Saunders JA. Do humans optimally integrate stereo and texture information for judgments of surface slant? Vision Res. 2003;43:2539–2558. - PubMed
-
- van Beers RJ, Sittig AC, Gon JJ. Integration of proprioceptive and visual position-information: An experimentally supported model. J Neurophysiol. 1999;81:1355–1364. - PubMed
-
- Ernst MO, Banks MS. Humans integrate visual and haptic information in a statistically optimal fashion. Nature. 2002;415:429–433. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

