A role for phosphatidic acid in COPI vesicle fission yields insights into Golgi maintenance
- PMID: 18776900
- PMCID: PMC2756218
- DOI: 10.1038/ncb1774
A role for phosphatidic acid in COPI vesicle fission yields insights into Golgi maintenance
Abstract
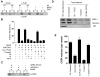
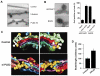
Proteins essential for vesicle formation by the Coat Protein I (COPI) complex are being identified, but less is known about the role of specific lipids. Brefeldin-A ADP-ribosylated substrate (BARS) functions in the fission step of COPI vesicle formation. Here, we show that BARS induces membrane curvature in cooperation with phosphatidic acid. This finding has allowed us to further delineate COPI vesicle fission into two sub-stages: 1) an earlier stage of bud-neck constriction, in which BARS and other COPI components are required, and 2) a later stage of bud-neck scission, in which phosphatidic acid generated by phospholipase D2 (PLD2) is also required. Moreover, in contrast to the disruption of the Golgi seen on perturbing the core COPI components (such as coatomer), inhibition of PLD2 causes milder disruptions, suggesting that such COPI components have additional roles in maintaining Golgi structure other than through COPI vesicle formation.
Figures



References
-
- Lee MC, Miller EA, Goldberg J, Orci L, Schekman R. Bi-directional protein transport between the ER and Golgi. Annu Rev Cell Dev Biol. 2004;20:87–123. - PubMed
-
- Rabouille C, Klumperman J. Opinion: The maturing role of COPI vesicles in intra-Golgi transport. Nat Rev Mol Cell Biol. 2005;6:812–7. - PubMed
-
- Nie Z, Hirsch DS, Randazzo PA. Arf and its many interactors. Curr Opin Cell Biol. 2003;15:396–404. - PubMed
-
- D'Souza-Schorey C, Chavrier P. ARF proteins: roles in membrane traffic and beyond. Nat Rev Mol Cell Biol. 2006;7:347–58. - PubMed
-
- Donaldson JG, Finazzi D, Klausner RD. Brefeldin A inhibits Golgi membrane-catalysed exchange of guanine nucleotide onto ARF protein. Nature. 1992;360:350–2. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

