Efficacy of Carraguard-based microbicides in vivo despite variable in vitro activity
- PMID: 18776937
- PMCID: PMC2525816
- DOI: 10.1371/journal.pone.0003162
Efficacy of Carraguard-based microbicides in vivo despite variable in vitro activity
Abstract
Anti-HIV microbicides are being investigated in clinical trials and understanding how promising strategies work, coincident with demonstrating efficacy in vivo, is central to advancing new generation microbicides. We evaluated Carraguard and a new generation Carraguard-based formulation containing the non-nucleoside reverse transcriptase inhibitor (NNRTI) MIV-150 (PC-817). Since dendritic cells (DCs) are believed to be important in HIV transmission, the formulations were tested for the ability to limit DC-driven infection in vitro versus vaginal infection of macaques with RT-SHIV (SIVmac239 bearing HIV reverse transcriptase). Carraguard showed limited activity against cell-free and mature DC-driven RT-SHIV infections and, surprisingly, low doses of Carraguard enhanced infection. However, nanomolar amounts of MIV-150 overcame enhancement and blocked DC-transmitted infection. In contrast, Carraguard impeded infection of immature DCs coincident with DC maturation. Despite this variable activity in vitro, Carraguard and PC-817 prevented vaginal transmission of RT-SHIV when applied 30 min prior to challenge. PC-817 appeared no more effective than Carraguard in vivo, due to the limited activity of a single dose of MIV-150 and the dominant barrier effect of Carraguard. However, 3 doses of MIV-150 in placebo gel at and around challenge limited vaginal infection, demonstrating the potential activity of a topically applied NNRTI. These data demonstrate discordant observations when comparing in vitro and in vivo efficacy of Carraguard-based microbicides, highlighting the difficulties in testing putative anti-viral strategies in vitro to predict in vivo activity. This work also underscores the potential of Carraguard-based formulations for the delivery of anti-viral drugs to prevent vaginal HIV infection.
Conflict of interest statement
Figures

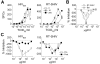
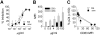
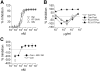


References
-
- DiClemente RJ, Wingood GM, Harrington KF, Lang DL, Davies SL, et al. Efficacy of an HIV prevention intervention for African American adolescent girls: a randomized controlled trial. Jama. 2004;292:171–179. - PubMed
-
- Gregson S, Garnett GP, Nyamukapa CA, Hallett TB, Lewis JJ, et al. HIV decline associated with behavior change in eastern Zimbabwe. Science. 2006;311:664–666. - PubMed
-
- Stoneburner RL, Low-Beer D. Population-level HIV declines and behavioral risk avoidance in Uganda. Science. 2004;304:714–718. - PubMed
-
- Shattock RJ, Moore JP. Inhibiting sexual transmission of HIV-1 infection. Nat Rev Microbiol. 2003;1:25–34. - PubMed
-
- From the Centers of Disease Control and Prevention. CDC statement on study results of product containing nonoxynol-9. Jama. 2000;284:1376. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

