Identification of proteins adducted by lipid peroxidation products in plasma and modifications of apolipoprotein A1 with a novel biotinylated phospholipid probe
- PMID: 18778096
- PMCID: PMC2664612
- DOI: 10.1021/pr8001222
Identification of proteins adducted by lipid peroxidation products in plasma and modifications of apolipoprotein A1 with a novel biotinylated phospholipid probe
Abstract
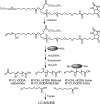
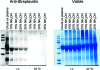
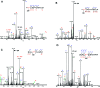
Reactive electrophiles generated by lipid peroxidation are thought to contribute to cardiovascular disease and other oxidative stress-related pathologies by covalently modifying proteins and affecting critical protein functions. The difficulty of capturing and analyzing the relatively small fraction of modified proteins complicates identification of the protein targets of lipid electrophiles. We recently synthesized a biotin-modified linoleoylglycerylphosphatidylcholine probe called PLPBSO ( Tallman et al. Chem. Res. Toxicol. 2007, 20, 227-234 ), which forms typical linoleate oxidation products and covalent adducts with model peptides and proteins. Supplementation of human plasma with PLPBSO followed by free radical oxidation resulted in covalent adduction of PLPBSO to plasma proteins, which were isolated with streptavidin and identified by liquid chromatography-tandem mass spectrometry (LC-MS-MS). Among the most highly modified proteins was apolipoprotein A1 (ApoA1), which is the core component of high density lipoprotein (HDL). ApoA1 phospholipid adduct sites were mapped by LC-MS-MS of tryptic peptides following mild base hydrolysis to release esterified phospholipid adducts. Several carboxylated adducts formed from phospholipid-esterified 9,12-dioxo-10( E)-dodecenoic acid (KODA), 9-hydroxy, 12-oxo-10( E)-dodecenoic acid (HODA), 7-oxoheptanoic acid, 8-oxooctanoic acid, and 9-oxononanoic acid were identified. Free radical oxidations of isolated HDL also generated adducts with 4-hydroxynonenal (HNE) and other noncarboxylated electrophiles, but these were only sporadically identified in the PLPBSO-adducted ApoA1, suggesting a low stoichiometry of modification in the phospholipid-adducted protein. Both phospholipid electrophiles and HNE adducted His162, which resides in an ApoA1 domain involved in the activation of Lecithin-cholesterol acyltransferase and maturation of the HDL particle. ApoA1 lipid electrophile adducts may affect protein functions and provide useful biomarkers for oxidative stress.
Figures


References
-
- Ames B. N. Dietary carcinogens and anticarcinogens. Oxygen radicals and degenerative diseases. Science 1983, 221, 1256–1264. - PubMed
-
- Halliwell B. Free radicals, antioxidants, and human disease: curiosity, cause, or consequence? [see comments]. Lancet 1994, 344(8924), 721–724. - PubMed
-
- Berliner J. A.; Watson A. D. A role for oxidized phospholipids in atherosclerosis. N. Engl. J. Med. 2005, 353(1), 9–11. - PubMed
-
- Butterfield D. A. Amyloid beta-peptide (1−42)-induced oxidative stress and neurotoxicity: implications for neurodegeneration in Alzheimer’s disease brain. A review. Free Radical Res. 2002, 36(12), 1307–1313. - PubMed
-
- Perry G.; Nunomura A.; Hirai K.; Zhu X.; Perez M.; Avila J.; Castellani R. J.; Atwood C. S.; Aliev G.; Sayre L. M.; Takeda A.; Smith M. A. Is oxidative damage the fundamental pathogenic mechanism of Alzheimer’s and other neurodegenerative diseases. Free Radical Biol. Med. 2002, 33(11), 1475–1479. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

