Genetic modification of adeno-associated viral vector type 2 capsid enhances gene transfer efficiency in polarized human airway epithelial cells
- PMID: 18778196
- PMCID: PMC6463989
- DOI: 10.1089/hum.2008.117
Genetic modification of adeno-associated viral vector type 2 capsid enhances gene transfer efficiency in polarized human airway epithelial cells
Abstract
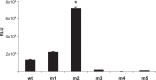
Cystic fibrosis (CF) is a common genetic disease characterized by defects in the expression of the CF transmembrane conductance regulator (CFTR) gene. Gene therapy offers better hope for the treatment of CF. Adeno-associated viral (AAV) vectors are capable of stable expression with low immunogenicity. Despite their potential in CF gene therapy, gene transfer efficiency by AAV is limited because of pathophysiological barriers in these patients. Although a few AAV serotypes have shown better transduction compared with the AAV2-based vectors, gene transfer efficiency in human airway epithelium has still not reached therapeutic levels. To engineer better AAV vectors for enhanced gene delivery in human airway epithelium, we developed and characterized mutant AAV vectors by genetic capsid modification, modeling the well-characterized AAV2 serotype. We genetically incorporated putative high-affinity peptide ligands to human airway epithelium on the GH loop region of AAV2 capsid protein. Six independent mutant AAV were constructed, containing peptide ligands previously reported to bind with high affinity for known and unknown receptors on human airway epithelial cells. The vectors were tested on nonairway cells and nonpolarized and polarized human airway epithelial cells for enhanced infectivity. One of the mutant vectors, with the peptide sequence THALWHT, not only showed the highest transduction in undifferentiated human airway epithelial cells but also indicated significant transduction in polarized cells. Interestingly, this modified vector was also able to infect cells independently of the heparan sulfate proteoglycan receptor. Incorporation of this ligand on other AAV serotypes, which have shown improved gene transfer efficiency in the human airway epithelium, may enhance the application of AAV vectors in CF gene therapy.
Conflict of interest statement
No competing financial interests exist.
Figures



Similar articles
-
Factors influencing adeno-associated virus-mediated gene transfer to human cystic fibrosis airway epithelial cells: comparison with adenovirus vectors.J Virol. 1998 Nov;72(11):8904-12. doi: 10.1128/JVI.72.11.8904-8912.1998. J Virol. 1998. PMID: 9765435 Free PMC article.
-
Generation of novel AAV variants by directed evolution for improved CFTR delivery to human ciliated airway epithelium.Mol Ther. 2009 Dec;17(12):2067-77. doi: 10.1038/mt.2009.155. Epub 2009 Jul 14. Mol Ther. 2009. PMID: 19603002 Free PMC article.
-
Spliceosome-mediated RNA trans-splicing with recombinant adeno-associated virus partially restores cystic fibrosis transmembrane conductance regulator function to polarized human cystic fibrosis airway epithelial cells.Hum Gene Ther. 2005 Sep;16(9):1116-23. doi: 10.1089/hum.2005.16.1116. Hum Gene Ther. 2005. PMID: 16149910
-
Novel Lung Tropic Adeno-Associated Virus Capsids for Therapeutic Gene Delivery.Hum Gene Ther. 2020 Sep;31(17-18):996-1009. doi: 10.1089/hum.2020.169. Epub 2020 Sep 8. Hum Gene Ther. 2020. PMID: 32799685 Review.
-
Gene therapy in cystic fibrosis.Chest. 2001 Sep;120(3 Suppl):124S-131S. doi: 10.1378/chest.120.3_suppl.124s. Chest. 2001. PMID: 11555567 Review.
Cited by
-
Perspective on Adeno-Associated Virus Capsid Modification for Duchenne Muscular Dystrophy Gene Therapy.Hum Gene Ther. 2015 Dec;26(12):786-800. doi: 10.1089/hum.2015.107. Epub 2015 Oct 15. Hum Gene Ther. 2015. PMID: 26414293 Free PMC article. Review.
-
Genetic therapies for cystic fibrosis lung disease.Hum Mol Genet. 2011 Apr 15;20(R1):R79-86. doi: 10.1093/hmg/ddr104. Epub 2011 Mar 21. Hum Mol Genet. 2011. PMID: 21422098 Free PMC article. Review.
-
Cystic Fibrosis Gene Therapy: Looking Back, Looking Forward.Genes (Basel). 2018 Nov 7;9(11):538. doi: 10.3390/genes9110538. Genes (Basel). 2018. PMID: 30405068 Free PMC article. Review.
-
Various AAV Serotypes and Their Applications in Gene Therapy: An Overview.Cells. 2023 Mar 1;12(5):785. doi: 10.3390/cells12050785. Cells. 2023. PMID: 36899921 Free PMC article. Review.
-
An acidic oligopeptide displayed on AAV2 improves axial muscle tropism after systemic delivery.Genet Vaccines Ther. 2012 Jun 18;10(1):3. doi: 10.1186/1479-0556-10-3. Genet Vaccines Ther. 2012. PMID: 22709483 Free PMC article.
References
-
- Aitken M.L., Moss R.B., Waltz D.A., Dovey M.E., Tonelli M.R., McNamara S.C., Gibson R.L., Ramsey B.W., Carter B.J., and Reynolds T.C. (2001). A phase I study of aerosolized administration of tgAAVCF to cystic fibrosis subjects with mild lung disease. Hum. Gene Ther. 12, 1907–1916 - PubMed
-
- Auger R., Robin P., Camier B., Vial G., Rossignol B., Tenu J.P., and Raymond M.N. (1999). Relationship between phosphatidic acid level and regulation of protein transit in colonic epithelial cell line HT29-cl19A. J. Biol. Chem. 274, 28652–28659 - PubMed
-
- Augeron C., and Laboisse C.L. (1984). Emergence of permanently differentiated cell clones in a human colonic cancer cell line in culture after treatment with sodium butyrate. Cancer Res. 44, 3961–3969 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

