The Purkinje cell; 2008 style
- PMID: 18778712
- PMCID: PMC4332524
- DOI: 10.1016/j.yjmcc.2008.08.001
The Purkinje cell; 2008 style
Abstract
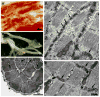
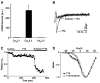
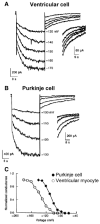
Cardiac Purkinje fibers, due to their unique anatomical location, cell structure and electrophysiologic characteristics, play an important role in cardiac conduction and arrhythmogenesis. Purkinje cell action potentials are longer than their ventricular counterpart, and display two levels of resting potential. Purkinje cells provide for rapid propagation of the cardiac impulse to ventricular cells and have pacemaker and triggered activity, which differs from ventricular cells. Additionally, a unique intracellular Ca2+ release coordination has been revealed recently for the normal Purkinje cell. However, since the isolation of single Purkinje cells is difficult, particularly in small animals, research using Purkinje cells has been restricted. This review concentrates on comparison of Purkinje and ventricular cells in the morphology of the action potential, ionic channel function and molecular determinants by summarizing our present day knowledge of Purkinje cells.
Figures
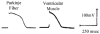
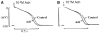
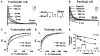
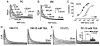
References
-
- Weerasooriya R, Hsu LF, Scavee C, Sanders P, Hocini M, Cabera JA, et al. Catheter Ablation of ventricular fibrillation in structurally normal hearts targeting the RVOT and Purkinje ectopy. Herz. 2003;28:598–606. - PubMed
-
- Lopera G, Stevenson WG, Soejima K, Maisel WH, Koplan B, Sapp JL, et al. Identification and ablation of three types of ventricular tachycardia involving the His–Purkinje system in patients with heart disease. J Cardiovasc Electr. 2004;15:52–8. - PubMed
-
- Kleber AG, Rudy Y. Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol Rev. 2004;84:431–88. - PubMed
-
- Di Maio A, Ter Keurs HEDJ, Franzini-Armstrong C. T-tubular profiles in Purkinje fibres of mammalian myocardium. J Musc Res Cell Motil. 2007;28:115–21. - PubMed
-
- Desplantez T, Dupont E, Severs N, Weingart R. Gap junction channels and cardiac impulse propagation. J Membr Biol. 2007;218:13–28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

