Synthesis and antiviral evaluation of acyclic azanucleosides developed from sulfanilamide as a lead structure
- PMID: 18778942
- PMCID: PMC7127773
- DOI: 10.1016/j.bmc.2008.08.041
Synthesis and antiviral evaluation of acyclic azanucleosides developed from sulfanilamide as a lead structure
Abstract
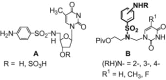
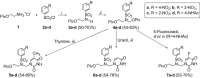
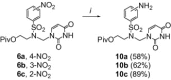
The acyclic azanucleosides with 2-, 3-, or 4-aminobenzenesulfonyl function at the nitrogen atom of the sugar mimic were prepared by coupling of 2-, 3-, or 4-nitro-N-(2-pivaloyloxyethyl)-N-(pivaloyloxymethyl)benzenesulfonamide with the silylated pyrimidine nucleobases followed by the reduction of the nitro group with sodium dithionite in aqueous solution or the palladium-catalysed transfer hydrogenation. The azanucleosides were evaluated for, but found to be devoid of, activity against several RNA- and DNA-viruses in vitro.
Figures
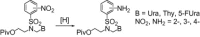
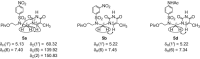


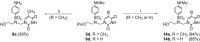
Similar articles
-
Synthesis and antiviral activity evaluation of acyclic 2'-azanucleosides bearing a phosphonomethoxy function in the side chain.Bioorg Med Chem. 2009 Jun 1;17(11):3756-62. doi: 10.1016/j.bmc.2009.04.054. Epub 2009 May 3. Bioorg Med Chem. 2009. PMID: 19442526 Free PMC article.
-
Synthesis and biological activities of (Z) and (E) alpha-ethenyl acyclonucleosides.Nucleosides Nucleotides Nucleic Acids. 2001 Aug;20(8):1439-47. doi: 10.1081/NCN-100105239. Nucleosides Nucleotides Nucleic Acids. 2001. PMID: 11554537
-
Synthesis of novel 6-azapyrimidine acyclic nucleoside analogues and antiviral evaluation.Nucleosides Nucleotides Nucleic Acids. 2010 Jul;29(7):535-41. doi: 10.1080/15257771003781634. Nucleosides Nucleotides Nucleic Acids. 2010. PMID: 20589572
-
The antiviral activity and mechanism of action of (S)-[3-hydroxy-2-(phosphonomethoxy)propyl] (HPMP) nucleosides.Antiviral Res. 2012 Nov;96(2):169-80. doi: 10.1016/j.antiviral.2012.08.010. Epub 2012 Sep 6. Antiviral Res. 2012. PMID: 22960154 Review.
-
Stealth nucleosides: mode of action and potential use in the treatment of viral diseases.BioDrugs. 2003;17(3):169-77. doi: 10.2165/00063030-200317030-00003. BioDrugs. 2003. PMID: 12749753 Review.
Cited by
-
Synthesis, structure, and antimicrobial activity of heterocyclic phenylsulfonyl- and 4-aminophenylsulfonyl-carboximidamides.Monatsh Chem. 2012;143(8):1161-1169. doi: 10.1007/s00706-012-0769-6. Epub 2012 May 15. Monatsh Chem. 2012. PMID: 26166869 Free PMC article.
-
Pharmaceutical and medicinal significance of sulfur (SVI)-Containing motifs for drug discovery: A critical review.Eur J Med Chem. 2019 Jan 15;162:679-734. doi: 10.1016/j.ejmech.2018.11.017. Epub 2018 Nov 22. Eur J Med Chem. 2019. PMID: 30496988 Free PMC article. Review.
-
Synthesis and antibacterial evaluation of quinoline-sulfonamide hybrid compounds: a promising strategy against bacterial resistance.RSC Adv. 2025 Jan 17;15(3):1680-1689. doi: 10.1039/d4ra05069j. eCollection 2025 Jan 16. RSC Adv. 2025. PMID: 39831044 Free PMC article.
-
Toxicity Assessment of an Anti-Cancer Drug of p-Toluene Sulfonamide in Zebrafish Larvae Based on Cardiovascular and Locomotion Activities.Biomolecules. 2022 Aug 10;12(8):1103. doi: 10.3390/biom12081103. Biomolecules. 2022. PMID: 36008997 Free PMC article.
-
Schiff bases of sulphonamides as a new class of antifungal agent against multidrug-resistant Candida auris.Microbiologyopen. 2021 Aug;10(4):e1218. doi: 10.1002/mbo3.1218. Microbiologyopen. 2021. PMID: 34459551 Free PMC article.
References
-
- Johnson A.A., Ray A.S., Hanes J., Suo Z., Colacino J.M., Anderson K.S., Johnson K.A. J. Biol. Chem. 2001;276:40847. - PubMed
- De Clercq E. J. Antimicrob. Chemother. 2003;51:1079. - PubMed
- Jeha S., Gandhi V., Chan K.W., McDonald L., Ramirez I., Madden R., Rytting M., Brandt M., Keating M., Plunkett W., Kantarjian H. Blood. 2004;103:784. - PubMed
-
- Gumina G., Olgen S., Chu C.K. In: Antiviral Nucleosides: Chiral Synthesis and Chemotherapy. Chu C.K., editor. Elsevier B.V.; 2003. pp. 161–169.
-
- Markham A.F., Newton C.R., Porter R.A., Sim I.S. Antiviral Res. 1982;2:319. For 5′-deoxy-5′-sulfonamidofuranosyl nucleosides, see: - PubMed
- Cosstick R., Jones A.S., Walker R.T. Tetrahedron. 1984;40:427.
- Elliott R.D., Brockman R.W., Montgomery J.A. J. Med. Chem. 1986;29:1052. - PubMed
- Sim I.S., Picton C., Cosstick R., Jones A.S., Walker R.T., Chamiec A.J. Nucleosides Nucleotides. 1988;7:129.
- Martin J.A., Duncan I.B., Hall M.J., Wong-Kai-In P., Lambert R.W., Thomas G.J. Nucleosides Nucleotides. 1989;8:753.
- Homma H., Watanabe Y., Abiru T., Murayama T., Nomura Y., Matsuda A. J. Med. Chem. 1992;35:2881. - PubMed
- Criton M., Dewyntert G., Aouf N., Montero J.-L., Imbach J.-L. Nucleosides Nucleotides. 1995;14:1795.
- Sharma M., Li Y.X., Ledvina M., Bobek M. Nucleosides Nucleotides. 1995;14:1831.
- Urjasz W., Celewicz L., Golankiewicz K. Nucleosides Nucleotides. 1996;15:1189.
- Martin J.A., Thomas G.J., Merret J.H., Lambert R.W., Bushnel D.J., Dundson S.J., Freeman A.C., Hopkins R.A., Johns I.R., Keech E., Simmonite H., Wong-Kai-In P., Holland M. Antiviral Chem. Chemother. 1998;1:1. - PubMed
- Scherman M.S., Winans K.A., Stern R.J., Jones V., Bertozzi C.R., McNeil M.R. Antimicrob. Agents Chemother. 2003;47:378. - PMC - PubMed
- Vanada J., Bennet E., Wilson D., Bishoff H., Barry C., Aldrich C. Org. Lett. 2006;8:4707. - PMC - PubMed
-
- Van Calenbergh S., Van Den Eeckhout E., Herdewijn P., De Bruyn A., Verlinde C., Hol W., Callens M., Van Aerschot A., Rozenski J. Helv. Chim. Acta. 1994;77:631. For 3′-deoxy-3′-sulfonamidofuranosyl nucleosides, see:
- Yamana K., Ohashi Y., Nunota K., Nakano H. Tetrahedron. 1997;53:4265.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

