Characterization of a unique ClpB protein of Mycoplasma pneumoniae and its impact on growth
- PMID: 18779336
- PMCID: PMC2573332
- DOI: 10.1128/IAI.00698-08
Characterization of a unique ClpB protein of Mycoplasma pneumoniae and its impact on growth
Abstract
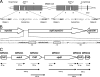
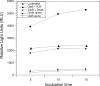
Mycoplasma pneumoniae accounts for 20 to 30% of all community-acquired pneumonia and has been associated with other airway pathologies, including asthma, and a range of extrapulmonary manifestations. Although the entire genomic sequence of M. pneumoniae has been completed, the functions of many of these genes in mycoplasma physiology are unknown. In this study, we focused on clpB, a well-known heat shock gene in other bacteria, to examine its role in mycoplasma growth. Transcriptional and translational analyses of heat shock in M. pneumoniae indicated that clpB is significantly upregulated, reinforcing its status as a critical responder to heat stress. Interestingly, M. pneumoniae ClpB does not use dual translational start points for ClpB synthesis, like other ClpB-characterized bacteria. Biochemical characterization of purified M. pneumoniae recombinant ClpB revealed casein- and lysine-independent ATPase activity and DnaK-DnaJ-GrpE-dependent chaperone activity. An M. pneumoniae mini-Tn4001-integrated, clpB-null mutant was impaired in its ability to replicate under permissive growth conditions, demonstrating the growth-promoting status of ClpB.
Figures




References
-
- Baseman, J. B. 1993. The cytadhesins of Mycoplasma pneumoniae and M. genitalium. Subcell. Biochem. 20243-259. - PubMed
-
- Beinker, P., S. Schlee, Y. Groemping, R. Seidel, and J. Reinstein. 2002. The N terminus of ClpB from Thermus thermophilus is not essential for the chaperone activity. J. Biol. Chem. 27747160-47166. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

