Ly6c+ "inflammatory monocytes" are microglial precursors recruited in a pathogenic manner in West Nile virus encephalitis
- PMID: 18779347
- PMCID: PMC2556789
- DOI: 10.1084/jem.20080421
Ly6c+ "inflammatory monocytes" are microglial precursors recruited in a pathogenic manner in West Nile virus encephalitis
Abstract
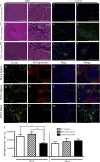
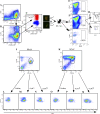
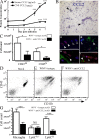
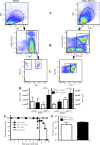
In a lethal West Nile virus (WNV) model, central nervous system infection triggered a threefold increase in CD45(int)/CD11b(+)/CD11c(-) microglia at days 6-7 postinfection (p.i.). Few microglia were proliferating, suggesting that the increased numbers were derived from a migratory precursor cell. Depletion of "circulating" (Gr1(-)(Ly6C(lo))CX3CR1(+)) and "inflammatory" (Gr1(hi)/Ly6C(hi)/CCR2(+)) classical monocytes during infection abrogated the increase in microglia. C57BL/6 chimeras reconstituted with cFMS-enhanced green fluorescent protein (EGFP) bone marrow (BM) showed large numbers of peripherally derived (GFP(+)) microglia expressing GR1(+)(Ly6C(+)) at day 7 p.i., suggesting that the inflammatory monocyte is a microglial precursor. This was confirmed by adoptive transfer of labeled BM (Ly6C(hi)/CD115(+)) or circulating inflammatory monocytes that trafficked to the WNV-infected brain and expressed a microglial phenotype. CCL2 is a chemokine that is highly expressed during WNV infection and important in inflammatory monocyte trafficking. Neutralization of CCL2 not only reduced the number of GFP(+) microglia in the brain during WNV infection but prolonged the life of infected animals. Therefore, CCL2-dependent inflammatory monocyte migration is critical for increases in microglia during WNV infection and may also play a pathogenic role during WNV encephalitis.
Figures




References
-
- Dalmau, I., J.M. Vela, B. Gonzalez, B. Finsen, and B. Castellano. 2003. Dynamics of microglia in the developing rat brain. J. Comp. Neurol. 458:144–157. - PubMed
-
- Fendrick, S.E., K.R. Miller, and W.J. Streit. 2005. Minocycline does not inhibit microglia proliferation or neuronal regeneration in the facial nucleus following crush injury. Neurosci. Lett. 385:220–223. - PubMed
-
- Lytle, J.M., and J.R. Wrathall. 2007. Glial cell loss, proliferation and replacement in the contused murine spinal cord. Eur. J. Neurosci. 25:1711–1724. - PubMed
-
- Pessac, B., I. Godin, and F. Alliot. 2001. Microglia: origin and development. Bull. Acad. Natl. Med. 185:337–346. - PubMed
-
- Djukic, M., A. Mildner, H. Schmidt, D. Czesnik, W. Bruck, J. Priller, R. Nau, and M. Prinz. 2006. Circulating monocytes engraft in the brain, differentiate into microglia and contribute to the pathology following meningitis in mice. Brain. 129:2394–2403. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

