Survival of the weakest: signaling aided by endosomes
- PMID: 18779366
- PMCID: PMC2528579
- DOI: 10.1083/jcb.200807165
Survival of the weakest: signaling aided by endosomes
Abstract
The tyrosine kinase receptor c-Met plays a key role in cell proliferation, morphogenesis, and motility in response to hepatocyte growth factor. C-Met is often altered in cancer and is a major target for therapeutic intervention. Despite knowing a great deal of the molecular machinery downstream of this receptor tyrosine kinase, the spatiotemporal regulation of c-Met signaling still remains elusive. In this issue of the Journal of Cell Biology, Kermorgant and Parker (Kermorgant, S. and P.J. Parker. 2008. J. Cell Biol. 182:855-863) provide evidence for a model in which the c-Met-activated STAT3 signal is mediated by endosomal trafficking. This study elegantly highlights how weak signals can be effectively transmitted to the nucleus by exploiting endosomal compartments, raising important mechanistic implications for the signaling research community.
Figures
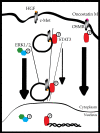
Comment on
-
Receptor trafficking controls weak signal delivery: a strategy used by c-Met for STAT3 nuclear accumulation.J Cell Biol. 2008 Sep 8;182(5):855-63. doi: 10.1083/jcb.200806076. J Cell Biol. 2008. PMID: 18779368 Free PMC article.
References
-
- Birchmeier, C., W. Birchmeier, E. Gherardi, and G.F. Vande Woude. 2003. Met, metastasis, motility and more. Nat. Rev. Mol. Cell Biol. 4:915–925. - PubMed
-
- Boccaccio, C., M. Ando, L. Tamagnone, A. Bardelli, P. Michieli, C. Battistini, and P.M. Comoglio. 1998. Induction of epithelial tubules by growth factor HGF depends on the STAT pathway. Nature. 391:285–288. - PubMed
-
- Comoglio, P.M., S. Giordano, and L. Trusolino. 2008. Drug development of MET inhibitors: targeting oncogene addiction and expedience. Nat. Rev. Drug Discov. 7:504–516. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

