Cardiolipin defines the interactome of the major ADP/ATP carrier protein of the mitochondrial inner membrane
- PMID: 18779372
- PMCID: PMC2528576
- DOI: 10.1083/jcb.200801152
Cardiolipin defines the interactome of the major ADP/ATP carrier protein of the mitochondrial inner membrane
Erratum in
- J Cell Biol. 2012 Jun 25;197(7):1029
Abstract
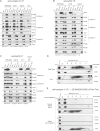
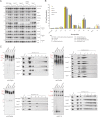
Defined mutations in the mitochondrial ADP/ATP carrier (AAC) are associated with certain types of progressive external ophthalmoplegia. AAC is required for oxidative phosphorylation (OXPHOS), and dysregulation of AAC has been implicated in apoptosis. Little is known about the AAC interactome, aside from a known requirement for the phospholipid cardiolipin (CL) and that it is thought to function as a homodimer. Using a newly developed dual affinity tag, we demonstrate that yeast AAC2 physically participates in several protein complexes of distinct size and composition. The respiratory supercomplex and several smaller AAC2-containing complexes, including other members of the mitochondrial carrier family, are identified here. In the absence of CL, most of the defined interactions are destabilized or undetectable. The absence of CL and/or AAC2 results in distinct yet additive alterations in respiratory supercomplex structure and respiratory function. Thus, a single lipid can significantly alter the functional interactome of an individual protein.
Figures





References
-
- Beyer, K., and B. Nuscher. 1996. Specific cardiolipin binding interferes with labeling of sulfhydryl residues in the adenosine diphosphate/adenosine triphosphate carrier protein from beef heart mitochondria. Biochemistry. 35:15784–15790. - PubMed
-
- Boumans, H., L.A. Grivell, and J.A. Berden. 1998. The respiratory chain in yeast behaves as a single functional unit. J. Biol. Chem. 273:4872–4877. - PubMed
-
- Chicco, A.J., and G.C. Sparagna. 2007. Role of cardiolipin alterations in mitochondrial dysfunction and disease. Am. J. Physiol. Cell Physiol. 292:C33–C44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

