TGF-beta and IL-6 signals modulate chromatin binding and promoter occupancy by acetylated FOXP3
- PMID: 18779564
- PMCID: PMC2544572
- DOI: 10.1073/pnas.0806726105
TGF-beta and IL-6 signals modulate chromatin binding and promoter occupancy by acetylated FOXP3
Abstract
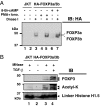
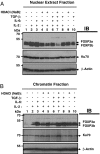
Expression of FOXP3, a potent gene-specific transcriptional repressor, in regulatory T cells is required to suppress autoreactive and alloreactive effector T cell function. Recent studies have shown that FOXP3 is an acetylated protein in a large nuclear complex and FOXP3 actively represses transcription by recruiting enzymatic corepressors, including histone modification enzymes. The mechanism by which extracellular stimuli regulate the FOXP3 complex ensemble is currently unknown. Although TGF-beta is known to induce murine FOXP3(+) Treg cells, TGF-beta in combination with IL-6 attenuates the induction of FOXP3 functional activities. Here we show that TCR stimuli and TGF-beta signals modulate the disposition of FOXP3 into different subnuclear compartments, leading to enhanced chromatin binding in human CD4(+)CD25(+) regulatory T cells. TGF-beta treatment increases the level of acetylated FOXP3 on chromatin and site-specific recruitment of FOXP3 on the human IL-2 promoter. However, the proinflammatory cytokine IL-6 down-regulates FOXP3 binding to chromatin in the presence of TGF-beta. Moreover, histone deacetylation inhibitor (HDACi) treatment abrogates the down-regulating effects of IL-6 and TGF-beta. These studies indicate that HDACi can enhance regulatory T cell function via promoting FOXP3 binding to chromatin even in a proinflammatory cellular microenvironment. Collectively, our data provide a framework of how different signals affect intranuclear redistribution, posttranslational modifications, and chromatin binding patterns of FOXP3.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. - PubMed
-
- Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. - PubMed
-
- Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: Regulatory T cell development and the forkhead family transcription factor Foxp3. Nat Immunol. 2005;6:331–337. - PubMed
-
- Brunkow ME, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

