Spatial organization of a replicating bacterial chromosome
- PMID: 18779567
- PMCID: PMC2544591
- DOI: 10.1073/pnas.0804982105
Spatial organization of a replicating bacterial chromosome
Abstract
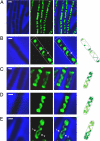
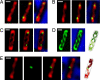
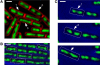
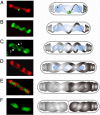
Emerging evidence indicates that the global organization of the bacterial chromosome is defined by its physical map. This architectural understanding has been gained mainly by observing the localization and dynamics of specific chromosomal loci. However, the spatial and temporal organization of the entire mass of newly synthesized DNA remains elusive. To visualize replicated DNA within living cells, we developed an experimental system in the bacterium Bacillus subtilis whereby fluorescently labeled nucleotides are incorporated into the chromosome as it is being replicated. Here, we present the first visualization of replication morphologies exhibited by the bacterial chromosome. At the start of replication, newly synthesized DNA is translocated via a helical structure from midcell toward the poles, where it accumulates. Next, additionally synthesized DNA forms a second, visually distinct helix that interweaves with the original one. In the final stage of replication, the space between the two helices is filled up with the very last synthesized DNA. This striking geometry provides insight into the three-dimensional conformation of the replicating chromosome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Cooper S, Helmstetter CE. Chromosome replication and the division cycle of Escherichia coli B/r. J Mol Biol. 1968;31:519–540. - PubMed
-
- Hazan R, Ben-Yehuda S. Resolving chromosome segregation in bacteria. J Mol Microbiol Biotechnol. 2006;11:126–139. - PubMed
-
- Hiraga S. Dynamic localization of bacterial and plasmid chromosomes. Annu Rev Genet. 2000;34:21–59. - PubMed
-
- Lemon KP, Grossman AD. The extrusion-capture model for chromosome partitioning in bacteria. Genes Dev. 2001;15:2031–2041. - PubMed
-
- Nielsen HJ, Li Y, Youngren B, Hansen FG, Austin S. Progressive segregation of the Escherichia coli chromosome. Mol Microbiol. 2006;61:383–393. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

