Cross-talk between GlcNAcylation and phosphorylation: site-specific phosphorylation dynamics in response to globally elevated O-GlcNAc
- PMID: 18779572
- PMCID: PMC2544533
- DOI: 10.1073/pnas.0806216105
Cross-talk between GlcNAcylation and phosphorylation: site-specific phosphorylation dynamics in response to globally elevated O-GlcNAc
Abstract
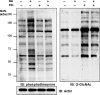
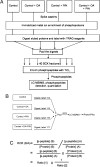
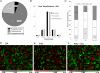
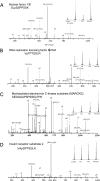
Protein GlcNAcylation serves as a nutrient/stress sensor to modulate the functions of many nuclear and cytoplasmic proteins. O-GlcNAc cycles on serine or threonine residues like phosphorylation, is nearly as abundant, and functions, at least partially, via its interplay with phosphorylation. Here, we describe changes in site-specific phosphorylation dynamics in response to globally elevated GlcNAcylation. By combining sequential phospho-residue enrichment, iTRAQ labeling, and high throughput mass spectrometric analyses, phosphorylation dynamics on 711 phosphopeptides were quantified. Based upon their insensitivity to phosphatase inhibition, we conclude that approximately 48% of these phosphorylation sites were not actively cycling in the conditions of the study. However, increased GlcNAcylation influenced phosphate stoichiometry at most of the sites that did appear to be actively cycling. Elevated GlcNAcylation resulted in lower phosphorylation at 280 sites and caused increased phosphorylation at 148 sites. Thus, the cross-talk or interplay between these two abundant posttranslational modifications is extensive, and may arises both by steric competition for occupancy at the same or proximal sites and by each modification regulating the other's enzymatic machinery. The phosphoproteome dynamics presented by this large set of quantitative data not only delineates the complex interplay between phosphorylation and GlcNAcyation, but also provides insights for more focused investigations of specific roles of O-GlcNAc in regulating protein functions and signaling pathways.
Conflict of interest statement
Conflict of interest statement: G.W.H. receives a share of royalty received by the university on sales of the CTD 110.6 antibody. Terms of this arrangement are managed by Johns Hopkins University School of Medicine.
Figures
References
-
- Hart G, Housley M, Slawson C. Cycling of O-linked β-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 2007;446:1017–1022. - PubMed
-
- Slawson C, Housley M, Hart G. O-GlcNAc cycling: How a single sugar post-translational modification is changing the way we think about signaling networks. J Cell Biochem. 2006;97:71–83. - PubMed
-
- Zachara N, Hart G. O-GlcNAc a sensor of cellular state: The role of nucleocytoplasmic glycosylation in modulating cellular function in response to nutrition and stress. Biochim Biophys Acta. 2004;1673:13–28. - PubMed
-
- Kreppel L, Hart G. Regulation of a cytosolic and nuclear O-GlcNAc transferase. Role of the tetratricopeptide repeats. J Biol Chem. 1999;274:32015–32022. - PubMed
-
- Gao Y, Wells L, Comer F, Parker G, Hart G. Dynamic O-glycosylation of nuclear and cytosolic proteins. Cloning and characterization of a neutral, cytosolic β-N-acetylglucosaminidase from human brain. J Biol Chem. 2000;276:9838–9845. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

