C-terminal peptides coassemble into Abeta42 oligomers and protect neurons against Abeta42-induced neurotoxicity
- PMID: 18779585
- PMCID: PMC2544597
- DOI: 10.1073/pnas.0807163105
C-terminal peptides coassemble into Abeta42 oligomers and protect neurons against Abeta42-induced neurotoxicity
Abstract
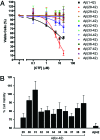
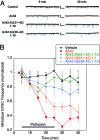
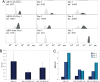
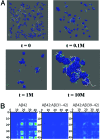
Alzheimer's disease (AD) is an age-related disorder that threatens to become an epidemic as the world population ages. Neurotoxic oligomers of Abeta42 are believed to be the main cause of AD; therefore, disruption of Abeta oligomerization is a promising approach for developing therapeutics for AD. Formation of Abeta42 oligomers is mediated by intermolecular interactions in which the C terminus plays a central role. We hypothesized that peptides derived from the C terminus of Abeta42 may get incorporated into oligomers of Abeta42, disrupt their structure, and thereby inhibit their toxicity. We tested this hypothesis using Abeta fragments with the general formula Abeta(x-42) (x = 28-39). A cell viability screen identified Abeta(31-42) as the most potent inhibitor. In addition, the shortest peptide, Abeta(39-42), also had high activity. Both Abeta(31-42) and Abeta(39-42) inhibited Abeta-induced cell death and rescued disruption of synaptic activity by Abeta42 oligomers at micromolar concentrations. Biophysical characterization indicated that the action of these peptides likely involved stabilization of Abeta42 in nontoxic oligomers. Computer simulations suggested a mechanism by which the fragments coassembled with Abeta42 to form heterooligomers. Thus, Abeta(31-42) and Abeta(39-42) are leads for obtaining mechanism-based drugs for treatment of AD using a systematic structure-activity approach.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer's disease. Alzheimer's Dementia. 2007;3:186–191. - PubMed
-
- Alzheimer Association. 2008 Alzheimer's disease facts and figures. Alzheimer's Dementia. 2008;4:110–133. - PubMed
-
- Hardy JA, Higgins GA. Alzheimer's disease: The amyloid cascade hypothesis. Science. 1992;256:184–185. - PubMed
-
- Kirkitadze MD, Bitan G, Teplow DB. Paradigm shifts in Alzheimer's disease and other neurodegenerative disorders: The emerging role of oligomeric assemblies. J Neurosci Res. 2002;69:567–577. - PubMed
-
- Walsh DM, Selkoe DJ. Aβ oligomers—A decade of discovery. J Neurochem. 2007;101:1172–1184. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

