Intraretinal signaling by ganglion cell photoreceptors to dopaminergic amacrine neurons
- PMID: 18779590
- PMCID: PMC2544598
- DOI: 10.1073/pnas.0803893105
Intraretinal signaling by ganglion cell photoreceptors to dopaminergic amacrine neurons
Abstract
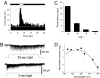
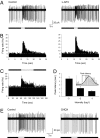
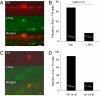
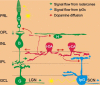
Retinal dopaminergic amacrine neurons (DA neurons) play a central role in reconfiguring retinal function according to prevailing illumination conditions, yet the mechanisms by which light regulates their activity are poorly understood. We investigated the means by which sustained light responses are evoked in DA neurons. Sustained light responses were driven by cationic currents and persisted in vitro and in vivo in the presence of L-AP4, a blocker of retinal ON-bipolar cells. Several characteristics of these L-AP4-resistant light responses suggested that they were driven by melanopsin-expressing intrinsically photosensitive retinal ganglion cells (ipRGCs), including long latencies, marked poststimulus persistence, and a peak spectral sensitivity of 478 nm. Furthermore, sustained DA neuron light responses, but not transient DA neuron responses, persisted in rod/cone degenerate retinas, in which ipRGCs account for virtually all remaining retinal phototransduction. Thus, ganglion-cell photoreceptors provide excitatory drive to DA neurons, most likely by way of the coramification of their dendrites and the processes of DA neurons in the inner plexiform layer. This unprecedented centrifugal outflow of ganglion-cell signals within the retina provides a novel basis for the restructuring of retinal circuits by light.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Dowling JE. The Retina: An Approachable Part of the Brain. Cambridge, MA: Harvard Univ Press; 1987.
-
- Dowling JE, Ehinger B. Synaptic organization of the amine-containing interplexiform cells of the goldfish and Cebus monkey retinas. Science. 1975;188:270–273. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 EY17137/EY/NEI NIH HHS/United States
- R01 EY017809/EY/NEI NIH HHS/United States
- R01 EY12793/EY/NEI NIH HHS/United States
- K99 EY018863/EY/NEI NIH HHS/United States
- F32 EY16678/EY/NEI NIH HHS/United States
- R56 EY009256/EY/NEI NIH HHS/United States
- EY9256/EY/NEI NIH HHS/United States
- R01 EY012793/EY/NEI NIH HHS/United States
- R01 EY009256/EY/NEI NIH HHS/United States
- R01 EY015815/EY/NEI NIH HHS/United States
- P30 EY008126/EY/NEI NIH HHS/United States
- F32 EY016678/EY/NEI NIH HHS/United States
- NS 035615/NS/NINDS NIH HHS/United States
- R01 NS035615/NS/NINDS NIH HHS/United States
- R01 EY017137/EY/NEI NIH HHS/United States
- P30-EY008126/EY/NEI NIH HHS/United States
- R01 EY15815/EY/NEI NIH HHS/United States
- R03 EY0107032/EY/NEI NIH HHS/United States
- K99 EY18863/EY/NEI NIH HHS/United States
- EY 17809/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

