Efficacy of RAD001 (everolimus) and octreotide LAR in advanced low- to intermediate-grade neuroendocrine tumors: results of a phase II study
- PMID: 18779618
- PMCID: PMC2653122
- DOI: 10.1200/JCO.2008.16.7858
Efficacy of RAD001 (everolimus) and octreotide LAR in advanced low- to intermediate-grade neuroendocrine tumors: results of a phase II study
Erratum in
- J Clin Oncol. 2008 Dec 1;26(34)5660.
Abstract
Purpose: Evaluate the activity of everolimus (RAD001) in combination with octreotide long-acting repeatable (LAR) in patients with advanced low- to intermediate-grade neuroendocrine tumors.
Methods: Treatment consisted of RAD001 5 mg/d (30 patients) or 10 mg/d (30 patients) and octreotide LAR 30 mg every 28 days. Thirty carcinoid and 30 islet cell patients were enrolled.
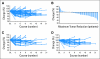
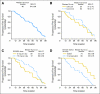
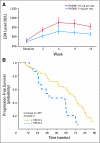
Results: Intent-to-treat response rate was 20%. Per protocol, there were 13 with partial responses (22%), 42 with stable disease (SD; 70%), and five patients with progressive disease (8%). Overall median progression-free survival (PFS) was 60 weeks. Median PFS for patients with known SD at entry was longer than for those who had progressive disease (74 v 50 weeks; P < .01). Median overall survival has not been reached. One-, 2-, and 3-year survival rates were 83%, 81%, and 78%, respectively. Among 37 patients with elevated chromogranin A, 26 (70%) achieved normalization or more than 50% reduction. Most common toxicity was mild aphthous ulceration. Grade 3/4 toxicities occurring in >or= 10% of patients included hypophosphatemia (11%), fatigue (11%), and diarrhea (11%). Treatment was associated with a dose-dependent rise in lactate dehydrogenase (LDH). Those with lower than 109 U/L rise in LDH at week 4 had shorter PFS (38 v 69 weeks; P = .01). Treatment was also associated with a decrease in proliferation marker Ki-67 among patients who underwent optional paired pre- and post-treatment biopsy (P = .04).
Conclusion: RAD001 at 5 or 10 mg/d was well tolerated in combination with octreotide LAR, with promising antitumor activity. Confirmatory studies are ongoing.
Figures

References
-
- Obendorfer S: Carcinoid of the small intestine [in German]. Frankf Z Pathol 1:425-429, 1907
-
- Kim do H, Nagano Y, Choi IS, et al: Allelic alterations in well-differentiated neuroendocrine tumors (carcinoid tumors) identified by genome-wide single nucleotide polymorphism analysis and comparison with pancreatic endocrine tumors. Genes Chromosomes Cancer 47:84-92, 2008 - PubMed
-
- Choi IS, Estecio MR, Nagano Y, et al: Hypomethylation of LINE-1 and Alu in well-differentiated neuroendocrine tumors (pancreatic endocrine tumors and carcinoid tumors). Mod Pathol 20:802-810, 2007 - PubMed
-
- Wang GG, Yao JC, Worah S, et al: Comparison of genetic alterations in neuroendocrine tumors: Frequent loss of chromosome 18 in ileal carcinoid tumors. Mod Pathol 18:1079-1087, 2005 - PubMed
-
- Modlin IM, Lye KD, Kidd M: A 5-decade analysis of 13,715 carcinoid tumors. Cancer 97:934-959, 2003 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

