Immunization with analog peptide in combination with CpG and montanide expands tumor antigen-specific CD8+ T cells in melanoma patients
- PMID: 18779741
- PMCID: PMC3901357
- DOI: 10.1097/CJI.0b013e318183af0b
Immunization with analog peptide in combination with CpG and montanide expands tumor antigen-specific CD8+ T cells in melanoma patients
Abstract
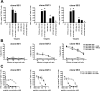
Analog peptides represent a promising tool to further optimize peptide-based vaccines in promoting the expansion of tumor antigen-specific cytotoxic T lymphocytes. Here, we report the results of a pilot trial designed to study the immunogenicity of the analog peptide NY-ESO-1 157-165V in combination with CpG 7909/PF3512676 and Montanide ISA 720 in patients with stage III/IV NY-ESO-1-expressing melanoma. Eight patients were immunized either with Montanide and CpG (arm 1, 3 patients); Montanide and peptide NY-ESO-1 157-165V (arm 2, 2 patients); or with Montanide, CpG, and peptide NY-ESO-1 157-165V (arm 3, 3 patients). Only the 3 patients immunized with Montanide, CpG, and peptide NY-ESO-1 157-165V in arm 3 developed a rapid increase of effector-memory NY-ESO-1-specific CD8+ T cells, detectable ex vivo. The majority of these cells exhibited an intermediate/late-stage differentiated phenotype (CD28-). Our study further demonstrated that our vaccine approach stimulated spontaneous tumor-reactive NY-ESO-1-specific CD8+ T cells in 2 patients with advanced disease, but failed to prime tumor-reactive NY-ESO-1-specific T cells in 1 patient with no spontaneously tumor-induced CD8+ T-cell responses to NY-ESO-1. Collectively, our data support the capability of the analog peptide NY-ESO-1 157-165V in combination with CpG and Montanide to promote the expansion of NY-ESO-1-specific CD8+ T cells in patients with advanced cancer. They also suggest that the presence of tumor-induced NY-ESO-1-specific T cells of well-defined clonotypes is critical for the expansion of tumor-reactive NY-ESO-1-specific CD8+ T cells after peptide-based vaccine strategies.
Figures
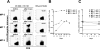


References
-
- Boon T, Coulie PG, Van den Eynde BJ, van der Bruggen P. Human T cell responses against melanoma. Annual review of immunology. 2006;24:175–208. - PubMed
-
- Gajewski TF. Identifying and overcoming immune resistance mechanisms in the melanoma tumor microenvironment. Clin Cancer Res. 2006;12:2326s–30s. - PubMed
-
- Baumgaertner P, Rufer N, Devevre E, et al. Ex vivo detectable human CD8 T-cell responses to cancer-testis antigens. Cancer research. 2006;66:1912–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

