Architectonic subdivisions of neocortex in the gray squirrel (Sciurus carolinensis)
- PMID: 18780299
- PMCID: PMC2908424
- DOI: 10.1002/ar.20758
Architectonic subdivisions of neocortex in the gray squirrel (Sciurus carolinensis)
Abstract
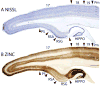
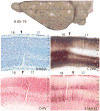
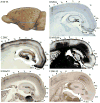
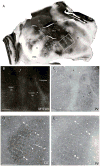
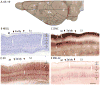
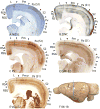
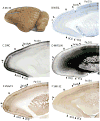
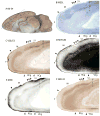
Squirrels are highly visual mammals with an expanded cortical visual system and a number of well-differentiated architectonic fields. To describe and delimit cortical fields, subdivisions of cortex were reconstructed from serial brain sections cut in the coronal, sagittal, or horizontal planes. Architectonic characteristics of cortical areas were visualized after brain sections were processed with immunohistochemical and histochemical procedures for revealing parvalbumin, calbindin, neurofilament protein, vesicle glutamate transporter 2, limbic-associated membrane protein, synaptic zinc, cytochrome oxidase, myelin or Nissl substance. In general, these different procedures revealed similar boundaries between areas, suggesting that functionally relevant borders were being detected. The results allowed a more precise demarcation of previously identified areas as well as the identification of areas that had not been previously described. Primary sensory cortical areas were characterized by sparse zinc staining of layer 4, as thalamocortical terminations lack zinc, as well as by layer 4 terminations rich in parvalbumin and vesicle glutamate transporter 2. Primary areas also expressed higher levels of cytochrome oxidase and myelin. Primary motor cortex was associated with large SMI-32 labeled pyramidal cells in layers 3 and 5. Our proposed organization of cortex in gray squirrels includes both similarities and differences to the proposed of cortex in other rodents such as mice and rats. The presence of a number of well-differentiated cortical areas in squirrels may serve as a guide to the identification of homologous fields in other rodents, as well as a useful guide in further studies of cortical organization and function.
Figures











Similar articles
-
Architectonic subdivisions of neocortex in the tree shrew (Tupaia belangeri).Anat Rec (Hoboken). 2009 Jul;292(7):994-1027. doi: 10.1002/ar.20916. Anat Rec (Hoboken). 2009. PMID: 19462403 Free PMC article.
-
An architectonic study of the neocortex of the short-tailed opossum (Monodelphis domestica).Brain Behav Evol. 2009;73(3):206-28. doi: 10.1159/000225381. Epub 2009 Jun 16. Brain Behav Evol. 2009. PMID: 19546531 Free PMC article.
-
Architectonic subdivisions of neocortex in the Galago (Otolemur garnetti).Anat Rec (Hoboken). 2010 Jun;293(6):1033-69. doi: 10.1002/ar.21109. Anat Rec (Hoboken). 2010. PMID: 20201060 Free PMC article.
-
Cortical connections of MT in four species of primates: areal, modular, and retinotopic patterns.Vis Neurosci. 1990 Aug;5(2):165-204. doi: 10.1017/s0952523800000213. Vis Neurosci. 1990. PMID: 2278944 Review.
-
The evolution of brains from early mammals to humans.Wiley Interdiscip Rev Cogn Sci. 2013 Jan;4(1):33-45. doi: 10.1002/wcs.1206. Epub 2012 Nov 8. Wiley Interdiscip Rev Cogn Sci. 2013. PMID: 23529256 Free PMC article. Review.
Cited by
-
The functional organization and cortical connections of motor cortex in squirrels.Cereb Cortex. 2012 Sep;22(9):1959-78. doi: 10.1093/cercor/bhr228. Epub 2011 Oct 20. Cereb Cortex. 2012. PMID: 22021916 Free PMC article.
-
Orientation selectivity in the visual cortex of the nine-banded armadillo.J Neurophysiol. 2017 Mar 1;117(3):1395-1406. doi: 10.1152/jn.00851.2016. Epub 2017 Jan 4. J Neurophysiol. 2017. PMID: 28053246 Free PMC article.
-
A connection to the past: Monodelphis domestica provides insight into the organization and connectivity of the brains of early mammals.J Comp Neurol. 2013 Dec 1;521(17):3877-97. doi: 10.1002/cne.23383. J Comp Neurol. 2013. PMID: 23784751 Free PMC article.
-
Escaping the nocturnal bottleneck, and the evolution of the dorsal and ventral streams of visual processing in primates.Philos Trans R Soc Lond B Biol Sci. 2022 Feb 14;377(1844):20210293. doi: 10.1098/rstb.2021.0293. Epub 2021 Dec 27. Philos Trans R Soc Lond B Biol Sci. 2022. PMID: 34957843 Free PMC article.
-
Architectonic features and relative locations of primary sensory and related areas of neocortex in mouse lemurs.J Comp Neurol. 2019 Feb 15;527(3):625-639. doi: 10.1002/cne.24419. Epub 2018 Apr 26. J Comp Neurol. 2019. PMID: 29484648 Free PMC article.
References
-
- Abplanalp P. Some subcortical connections of the visual system in tree shrews and squirrels. Brain Behav Evol. 1970;3(1):155–168. - PubMed
-
- Allman JM, Kaas JH. Representation of the visual field in striate and adjoining cortex of the owl monkey (Aotus trivirgatus) Brain Res. 1971;35(1):89–106. - PubMed
-
- Annese J, Pitiot A, Dinov ID, Toga AW. A myelo-architectonic method for the structural classification of cortical areas. Neuroimage. 2004;21(1):15–26. - PubMed
-
- Avendaño C, Verdu A. Area 3a in the cat. I. A reevaluation of its location and architecture on the basis of Nissl, myelin, acetylcholinesterase, and cytochrome oxidase staining. J Comp Neurol. 1992;321(3):357–72. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

