Ciglitazone, a PPARgamma agonist, ameliorates diabetic nephropathy in part through homocysteine clearance
- PMID: 18780770
- PMCID: PMC2584817
- DOI: 10.1152/ajpendo.90534.2008
Ciglitazone, a PPARgamma agonist, ameliorates diabetic nephropathy in part through homocysteine clearance
Abstract
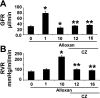
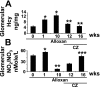
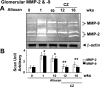
Diabetes and hyperhomocysteinemia (HHcy) are two independent risk factors for glomeruloslerosis and renal insufficiency. Although PPARgamma agonists such as ciglitazone (CZ) are known to modulate diabetic nephropathy, the role of CZ in diabetes-associated HHcy and renopathy is incompletely defined. We tested the hypothesis that induction of PPARgamma by CZ decreases tissue Hcy level; this provides a protective role against diabetic nephropathy. C57BL/6J mice were administered alloxan to create diabetes. Mice were grouped to 0, 1, 10, 12, and 16 wk of treatment; only 12- and 16-wk animals received CZ in drinking water after a 10-wk alloxan treatment. In diabetes, PPARgamma cDNA, mRNA, and protein expression were repressed, whereas an increase in plasma and glomerular Hcy levels was observed. CZ normalized PPARgamma mRNA and protein expression and glomerular level of Hcy, whereas plasma level of Hcy remained unchanged. GFR was dramatically increased at 1-wk diabetic induction, followed by hypofiltration at 10 wk, and was normalized by CZ treatment. This result corroborated with glomerular and preglomerular arteriole histology. A steady-state increase of RVR in diabetic mice became normal with CZ treatment. CZ ameliorated decrease bioavailability of NO in the diabetic animal. Glomerular MMP-2 and MMP-9 activities as well as TIMP-1 expression were increased robustly in diabetic mice and normalized with CZ treatment. Interestingly, TIMP-4 expression was opposite to that of TIMP-1 in diabetic and CZ-treated groups. These results suggested that diabetic nephropathy exacerbated glomerular tissue level of Hcy, and this caused further deterioration of glomerulus. CZ, however, protected diabetic nephropathy in part by activating PPARgamma and clearing glomerular tissue Hcy.
Figures






References
-
- Bissonnette R, Treacy E, Rozen R, Boucher B, Cohn JS, Genest J Jr. Fenofibrate raises plasma homocysteine levels in the fasted and fed states. Atherosclerosis 155: 455–462, 2001. - PubMed
-
- Buckingham RE, Al-Barazanji KA, Toseland CD, Slaughter M, Connor SC, West A, Bond B, Turner NC, Clapham JC. Peroxisome proliferator-activated receptor-gamma agonist, rosiglitazone, protects against nephropathy and pancreatic islet abnormalities in Zucker fatty rats. Diabetes 47: 1326–1334, 1998. - PubMed
-
- Buysschaert M, Dramais AS, Wallemacq PE, Hermans MP. Hyperhomocysteinemia in type 2 diabetes: relationship to macroangiopathy, nephropathy, and insulin resistance. Diabetes Care 23: 1816–1822, 2000. - PubMed
-
- Calkin AC, Cooper ME, Jandeleit-Dahm KA, Allen TJ. Gemfibrozil decreases atherosclerosis in experimental diabetes in association with a reduction in oxidative stress and inflammation. Diabetologia 49: 766–774, 2006. - PubMed
-
- Calkin AC, Giunti S, Jandeleit-Dahm KA, Allen TJ, Cooper ME, Thomas MC. PPAR-α and -γ agonists attenuate diabetic kidney disease in the apolipoprotein E knockout mouse. Nephrol Dial Transplant 21: 2399–2405, 2006. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

