Development of hippocampal mossy fiber synaptic outputs by new neurons in the adult brain
- PMID: 18780780
- PMCID: PMC2544594
- DOI: 10.1073/pnas.0806658105
Development of hippocampal mossy fiber synaptic outputs by new neurons in the adult brain
Erratum in
- Proc Natl Acad Sci U S A. 2008 Nov 25;105(47):18643
Abstract
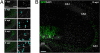
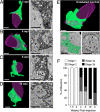
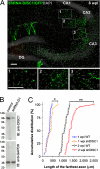
New neurons are continuously generated in restricted regions of the adult mammalian brain. Although these adult-born neurons have been shown to receive synaptic inputs, little is known about their synaptic outputs. Using retrovirus-mediated birth-dating and labeling in combination with serial section electron microscopic reconstruction, we report that mossy fiber en passant boutons of adult-born dentate granule cells form initial synaptic contacts with CA3 pyramidal cells within 2 weeks after their birth and reach morphologic maturity within 8 weeks in the adult hippocampus. Knockdown of Disrupted-in-Schizophrenia-1 (DISC1) in newborn granule cells leads to defects in axonal targeting and development of synaptic outputs in the adult brain. Together with previous reports of synaptic inputs, these results demonstrate that adult-born neurons are fully integrated into the existing neuronal circuitry. Our results also indicate a role for DISC1 in presynaptic development and may have implications for the etiology of schizophrenia and related mental disorders.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


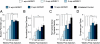
References
-
- Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. - PubMed
-
- Lledo PM, Alonso M, Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci. 2006;7:179–193. - PubMed
-
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG024984/AG/NIA NIH HHS/United States
- AG024984/AG/NIA NIH HHS/United States
- NS048271/NS/NINDS NIH HHS/United States
- R01 HD045757/HD/NICHD NIH HHS/United States
- MH084018/MH/NIMH NIH HHS/United States
- R56 NS047344/NS/NINDS NIH HHS/United States
- R01 NS048271/NS/NINDS NIH HHS/United States
- R01 NS047344/NS/NINDS NIH HHS/United States
- NS047344/NS/NINDS NIH HHS/United States
- HD045757/HD/NICHD NIH HHS/United States
- P20 MH084018/MH/NIMH NIH HHS/United States
- T32 EY017203/EY/NEI NIH HHS/United States
- R37 NS047344/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

