Memory CD8 T cell responses exceeding a large but definable threshold provide long-term immunity to malaria
- PMID: 18780790
- PMCID: PMC2544571
- DOI: 10.1073/pnas.0805452105
Memory CD8 T cell responses exceeding a large but definable threshold provide long-term immunity to malaria
Abstract
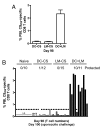
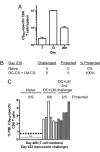
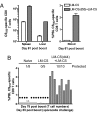
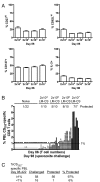
Infection of mice with sporozoites of Plasmodium berghei or Plasmodium yoelii has been used extensively to evaluate liver-stage protection by candidate preerythrocytic malaria vaccines. Unfortunately, repeated success of such vaccines in mice has not translated readily to effective malaria vaccines in humans. Thus, mice may be used better as models to dissect basic parameters required for immunity to Plasmodium-infection than as preclinical vaccine models. In turn, this basic information may aid in the rational design of malaria vaccines. Here, we describe a model of circumsporozoite-specific memory CD8 T cell generation that protects mice against multiple P. berghei sporozoite challenges for at least 19 months. Using this model we defined a threshold frequency of memory CD8 T cells in the blood that predicts long-term sterilizing immunity against liver-stage infection. Importantly, the number of Plasmodium-specific memory CD8 T cells required for immunity greatly exceeds the number required for resistance to other pathogens. In addition, this model allowed us to identify readily individual immunized mice that exceed or fall below the protective threshold before infection, information that should greatly facilitate studies to dissect basic mechanisms of protective CD8 T cell memory against liver-stage Plasmodium infection. Furthermore, the extremely large threshold in memory CD8 T cell frequencies required for long-term protection in mice may have important implications for development of effective malaria vaccines.
Conflict of interest statement
Conflict of interest statement: P.L. is an employee of ANZA Therapeutics, Inc, which owns intellectual property covering the compositions and methods described in this manuscript. In addition, ANZA employees hold stock and/or stock options in the company. The remaining authors disclose no known financial conflicts.
Figures

References
-
- Greenwood B, Mutabingwa T. Malaria in 2002. Nature. 2002;415:670–672. - PubMed
-
- Nussenzweig RS, Vanderberg J, Most H, Orton C. Protective immunity produced by the injection of x-irradiated sporozoites of Plasmodium berghei. Nature. 1967;216:160–162. - PubMed
-
- Tarun AS, et al. Protracted sterile protection with Plasmodium yoelii pre-erythrocytic genetically attenuated parasite malaria vaccines is independent of significant liver-stage persistence and is mediated by CD8+ T cells. J Infect Dis. 2007;196:608–616. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

