Preventing serpin aggregation: the molecular mechanism of citrate action upon antitrypsin unfolding
- PMID: 18780818
- PMCID: PMC2590919
- DOI: 10.1110/ps.037234.108
Preventing serpin aggregation: the molecular mechanism of citrate action upon antitrypsin unfolding
Abstract
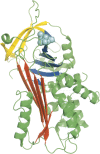
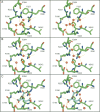
The aggregation of antitrypsin into polymers is one of the causes of neonatal hepatitis, cirrhosis, and emphysema. A similar reaction resulting in disease can occur in other human serpins, and collectively they are known as the serpinopathies. One possible therapeutic strategy involves inhibiting the conformational changes involved in antitrypsin aggregation. The citrate ion has previously been shown to prevent antitrypsin aggregation and maintain the protein in an active conformation; its mechanism of action, however, is unknown. Here we demonstrate that the citrate ion prevents the initial misfolding of the native state to a polymerogenic intermediate in a concentration-dependent manner. Furthermore, we have solved the crystal structure of citrate bound to antitrypsin and show that a single citrate molecule binds in a pocket between the A and B beta-sheets, a region known to be important in maintaining antitrypsin stability.
Figures


Similar articles
-
Defining the mechanism of polymerization in the serpinopathies.Proc Natl Acad Sci U S A. 2010 Oct 5;107(40):17146-51. doi: 10.1073/pnas.1004785107. Epub 2010 Sep 20. Proc Natl Acad Sci U S A. 2010. PMID: 20855577 Free PMC article.
-
The roles of helix I and strand 5A in the folding, function and misfolding of α1-antitrypsin.PLoS One. 2013;8(1):e54766. doi: 10.1371/journal.pone.0054766. Epub 2013 Jan 29. PLoS One. 2013. PMID: 23382962 Free PMC article.
-
Kinetic instability of the serpin Z alpha1-antitrypsin promotes aggregation.J Mol Biol. 2010 Feb 19;396(2):375-83. doi: 10.1016/j.jmb.2009.11.048. Epub 2009 Nov 26. J Mol Biol. 2010. PMID: 19944704
-
Wild-type alpha 1-antitrypsin is in the canonical inhibitory conformation.J Mol Biol. 1998 Jan 23;275(3):419-25. doi: 10.1006/jmbi.1997.1458. J Mol Biol. 1998. PMID: 9466920 Review.
-
Practical genetics: alpha-1-antitrypsin deficiency and the serpinopathies.Eur J Hum Genet. 2004 Mar;12(3):167-72. doi: 10.1038/sj.ejhg.5201127. Eur J Hum Genet. 2004. PMID: 14694355 Review.
Cited by
-
Development of a small molecule that corrects misfolding and increases secretion of Z α1 -antitrypsin.EMBO Mol Med. 2021 Mar 5;13(3):e13167. doi: 10.15252/emmm.202013167. Epub 2021 Jan 29. EMBO Mol Med. 2021. PMID: 33512066 Free PMC article.
-
Discovery of an inhibitor of Z-alpha1 antitrypsin polymerization.PLoS One. 2015 May 11;10(5):e0126256. doi: 10.1371/journal.pone.0126256. eCollection 2015. PLoS One. 2015. PMID: 25961288 Free PMC article.
-
Therapeutic target-site variability in α1-antitrypsin characterized at high resolution.Acta Crystallogr Sect F Struct Biol Cryst Commun. 2011 Dec 1;67(Pt 12):1492-7. doi: 10.1107/S1744309111040267. Epub 2011 Nov 25. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2011. PMID: 22139150 Free PMC article.
-
Structural and functional characterization of a highly specific serpin in the insect innate immunity.J Biol Chem. 2011 Jan 14;286(2):1567-75. doi: 10.1074/jbc.M110.144006. Epub 2010 Nov 3. J Biol Chem. 2011. PMID: 21047786 Free PMC article.
-
Smoothing a rugged protein folding landscape by sequence-based redesign.Sci Rep. 2016 Sep 26;6:33958. doi: 10.1038/srep33958. Sci Rep. 2016. PMID: 27667094 Free PMC article.
References
-
- Bird P.I., Pak S.C., Worrall D.M., Bottomley S.P. Production of recombinant serpins in Escherichia coli . Methods. 2004;32:169–176. - PubMed
-
- Bottomley S.P., Stone S.R. Protein engineering of chimeric serpins: An investigation into effects of the serpin scaffold and reactive centre loop length. Protein Eng. 1998;11:1243–1247. - PubMed
-
- Cabrita L.D., Dai W., Bottomley S.P. Different conformational changes within the F-helix occur during serpin folding, polymerization, and proteinase inhibition. Biochemistry. 2004;43:9834–9839. - PubMed
-
- Carrell R.W., Lomas D.A. Conformational disease. Lancet. 1997;350:134–138. - PubMed
-
- Chow M.K., Devlin G.L., Bottomley S.P. Osmolytes as modulators of conformational changes in serpins. Biol. Chem. 2001;382:1593–1599. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources

