Cross-talk of GATA-1 and P-TEFb in megakaryocyte differentiation
- PMID: 18780834
- PMCID: PMC2597596
- DOI: 10.1182/blood-2008-03-145722
Cross-talk of GATA-1 and P-TEFb in megakaryocyte differentiation
Abstract
The transcription factor GATA-1 participates in programming the differentiation of multiple hematopoietic lineages. In megakaryopoiesis, loss of GATA-1 function produces complex developmental abnormalities and underlies the pathogenesis of megakaryocytic leukemia in Down syndrome. Its distinct functions in megakaryocyte and erythroid maturation remain incompletely understood. In this study, we identified functional and physical interaction of GATA-1 with components of the positive transcriptional elongation factor P-TEFb, a complex containing cyclin T1 and the cyclin-dependent kinase 9 (Cdk9). Megakaryocytic induction was associated with dynamic changes in endogenous P-TEFb composition, including recruitment of GATA-1 and dissociation of HEXIM1, a Cdk9 inhibitor. shRNA knockdowns and pharmacologic inhibition both confirmed contribution of Cdk9 activity to megakaryocytic differentiation. In mice with megakaryocytic GATA-1 deficiency, Cdk9 inhibition produced a fulminant but reversible megakaryoblastic disorder reminiscent of the transient myeloproliferative disorder of Down syndrome. P-TEFb has previously been implicated in promoting elongation of paused RNA polymerase II and in programming hypertrophic differentiation of cardiomyocytes. Our results offer evidence for P-TEFb cross-talk with GATA-1 in megakaryocytic differentiation, a program with parallels to cardiomyocyte hypertrophy.
Figures
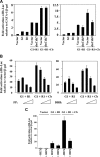
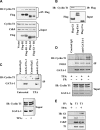
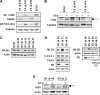
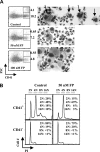
 ). (B) Ploidy analysis of CD41+ and CD41− cells within the cultures. Cells were stained with FITC-anti-CD41 and propidium iodide, followed by FACS analysis and quantitation with FlowJo software.
). (B) Ploidy analysis of CD41+ and CD41− cells within the cultures. Cells were stained with FITC-anti-CD41 and propidium iodide, followed by FACS analysis and quantitation with FlowJo software.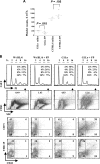
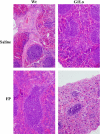
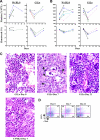
Comment in
-
GATA-1: one TEF customer.Blood. 2008 Dec 15;112(13):4786-7. doi: 10.1182/blood-2008-10-181743. Blood. 2008. PMID: 19064734 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

