Cell-permeable peptide Tat-PSD-95 PDZ2 inhibits chronic inflammatory pain behaviors in mice
- PMID: 18781143
- PMCID: PMC2747045
- DOI: 10.1038/mt.2008.192
Cell-permeable peptide Tat-PSD-95 PDZ2 inhibits chronic inflammatory pain behaviors in mice
Abstract
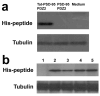
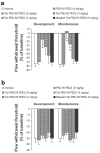
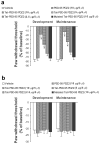
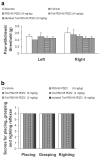
Inflammatory conditions can lead to persistent debilitating pain, and the activation of N-methyl-D-aspartate receptors (NMDARs) has been shown to play an important role in the processing of inflammatory pain. Postsynaptic density protein-95 (PSD-95), a scaffolding protein, has been identified to interact with NMDARs at neuronal synapses of the central nervous system (CNS). However, the role of these interactions in the central sensitization of nociceptive processing has not been defined. In this study, we investigated the effect of disrupting NMDAR/PSD-95 interactions on chronic inflammatory pain behaviors. We constructed a fusion peptide, Tat-PSD-95 PDZ2, comprising the second PDZ domain of PSD-95, to disrupt specifically NMDARs/PSD-95 protein interactions. Western blot analysis showed that Tat-PSD-95 PDZ2 intraperitoneally injected into mice was delivered intracellularly into neurons in the CNS. By in vitro and in vivo binding assays, we found that the Tat-PSD-95 PDZ2 dose dependently inhibited the interactions between NMDARs and PSD-95. Furthermore, behavioral testing showed that mice given Tat-PSD-95 PDZ2 exhibited significantly reduced complete Freund's adjuvant (CFA)-induced chronic inflammatory pain behaviors compared to the vehicle-treated group. Our results indicate that by disrupting NMDAR/PSD-95 protein interactions, the cell-permeable fusion peptide Tat-PSD-95 PDZ2 provides a new target and approach for chronic inflammatory pain therapy.
Figures

References
-
- Garry MG, Malik S, Yu J, Davis MA, Yang J. Knock down of spinal NMDA receptors reduces NMDA and formalin evoked behaviors in rat. Neuroreport. 2000;11:49–55. - PubMed
-
- Mao J, Price DD, Hayes RL, Lu J, Mayer DJ. Differential roles of NMDA and non-NMDA receptor activation in induction and maintenance of thermal hyperalgesia in rats with painful peripheral mononeuropathy. Brain Res. 1992;598:271–278. - PubMed
-
- Ren K, Hylden JL, Williams GM, Ruda MA, Dubner R. The effects of a non-competitive NMDA receptor antagonist, MK-801, on behavioral hyperalgesia and dorsal horn neuronal activity in rats with unilateral inflammation. Pain. 1992;50:331–344. - PubMed
-
- Wei F, Wang GD, Kerchner GA, Kim SJ, Xu HM, Chen ZF, et al. Genetic enhancement of inflammatory pain by forebrain NR2B overexpression. Nat Neurosci. 2001;4:164–169. - PubMed
-
- Christopherson KS, Hillier BJ, Lim WA, Bredt DS. PSD-95 assembles a ternary complex with the N-methyl-D-aspartic acid receptor and a bivalent neuronal NO synthase PDZ domain. J Biol Chem. 1999;274:27467–27473. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

