SIRT3 interacts with the daf-16 homolog FOXO3a in the mitochondria, as well as increases FOXO3a dependent gene expression
- PMID: 18781224
- PMCID: PMC2532794
- DOI: 10.7150/ijbs.4.291
SIRT3 interacts with the daf-16 homolog FOXO3a in the mitochondria, as well as increases FOXO3a dependent gene expression
Abstract
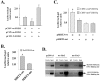
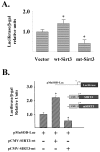
Cellular longevity is a complex process relevant to age-related diseases including but not limited to chronic illness such as diabetes and metabolic syndromes. Two gene families have been shown to play a role in the genetic regulation of longevity; the Sirtuin and FOXO families. It is also established that nuclear Sirtuins interact with and under specific cellular conditions regulate the activity of FOXO gene family proteins. Thus, we hypothesize that a mitochondrial Sirtuin (SIRT3) might also interact with and regulate the activity of the FOXO proteins. To address this we used HCT116 cells overexpressing either wild-type or a catalytically inactive dominant negative SIRT3. For the first time we establish that FOXO3a is also a mitochondrial protein and forms a physical interaction with SIRT3 in mitochondria. Overexpression of a wild-type SIRT3 gene increase FOXO3a DNA-binding activity as well as FOXO3a dependent gene expression. Biochemical analysis of HCT116 cells over expressing the deacetylation mutant, as compared to wild-type SIRT3 gene, demonstrated an overall oxidized intracellular environment, as monitored by increase in intracellular superoxide and oxidized glutathione levels. As such, we propose that SIRT3 and FOXO3a comprise a potential mitochondrial signaling cascade response pathway.
Keywords: FOXO3a; SIRT3; Superoxide; gene expression.
Conflict of interest statement
Conflict of Interest: The authors have declared that no conflict of interest exists.
Figures


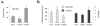
References
-
- Kaeberlein M, Jegalian B, McVey M. AGEID: a database of aging genes and interventions. Mechanisms of ageing and development. 2002;123(8):1115–9. - PubMed
-
- Guarente L. Calorie restriction and SIR2 genes--towards a mechanism. Mechanisms of ageing and development. 2005;126(9):923–8. - PubMed
-
- Okawara M, Katsuki H, Kurimoto E, Shibata H, Kume T, Akaike A. Resveratrol protects dopaminergic neurons in midbrain slice culture from multiple insults. Biochem Pharmacol. 2007;73(4):550–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

