Toll-like receptors at the ocular surface
- PMID: 18781257
- PMCID: PMC3403823
- DOI: 10.1016/s1542-0124(12)70279-3
Toll-like receptors at the ocular surface
Abstract
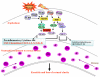
The Toll-like receptor (TLR) family of pathogen recognition molecules has an important role in recognizing microbial pathogens and microbial breakdown products. Activation of TLRs in the corneal epithelium induces CXC chemokine production and recruitment of neutrophils to the corneal stroma. Although essential for pathogen killing, neutrophils can cause extensive tissue damage, leading to visual impairment and blindness. In this review, we examine the role of TLRs in microbial keratitis and in noninfectious corneal inflammation, most commonly associated with contact lens wear. we present recent findings on TLR signaling pathways in the cornea, including MyD88- and TRIF-dependent responses and discuss the role of resident macrophages and dendritic cells. Finally, we examine the potential for targeting the TLR pathway as a potential therapeutic intervention for microbial keratitis and contact lens-associated corneal inflammation.
Figures


Similar articles
-
Activation of toll-like receptor (TLR)2, TLR4, and TLR9 in the mammalian cornea induces MyD88-dependent corneal inflammation.Invest Ophthalmol Vis Sci. 2005 Feb;46(2):589-95. doi: 10.1167/iovs.04-1077. Invest Ophthalmol Vis Sci. 2005. PMID: 15671286
-
Host defense at the ocular surface.Int Rev Immunol. 2013 Feb;32(1):4-18. doi: 10.3109/08830185.2012.749400. Int Rev Immunol. 2013. PMID: 23360155 Free PMC article. Review.
-
Staphylococcus aureus-induced corneal inflammation is dependent on Toll-like receptor 2 and myeloid differentiation factor 88.Infect Immun. 2006 Sep;74(9):5325-32. doi: 10.1128/IAI.00645-06. Infect Immun. 2006. PMID: 16926427 Free PMC article.
-
MyD88 functions as a negative regulator of TLR3/TRIF-induced corneal inflammation by inhibiting activation of c-Jun N-terminal kinase.J Biol Chem. 2008 Feb 15;283(7):3988-96. doi: 10.1074/jbc.M707264200. Epub 2007 Dec 5. J Biol Chem. 2008. PMID: 18057004
-
Pseudomonas aeruginosa infection and inflammation during contact lens wear: a review.Optom Vis Sci. 2007 Apr;84(4):273-8. doi: 10.1097/OPX.0b013e3180439c3e. Optom Vis Sci. 2007. PMID: 17435510 Review.
Cited by
-
MyD88 contribution to ocular surface homeostasis.PLoS One. 2017 Aug 10;12(8):e0182153. doi: 10.1371/journal.pone.0182153. eCollection 2017. PLoS One. 2017. PMID: 28796783 Free PMC article.
-
When Clarity Is Crucial: Regulating Ocular Surface Immunity.Trends Immunol. 2018 Apr;39(4):288-301. doi: 10.1016/j.it.2017.11.007. Epub 2017 Dec 14. Trends Immunol. 2018. PMID: 29248310 Free PMC article. Review.
-
A novel murine model for contact lens wear reveals clandestine IL-1R dependent corneal parainflammation and susceptibility to microbial keratitis upon inoculation with Pseudomonas aeruginosa.Ocul Surf. 2019 Jan;17(1):119-133. doi: 10.1016/j.jtos.2018.11.006. Epub 2018 Nov 12. Ocul Surf. 2019. PMID: 30439473 Free PMC article.
-
The cereus matter of Bacillus endophthalmitis.Exp Eye Res. 2020 Apr;193:107959. doi: 10.1016/j.exer.2020.107959. Epub 2020 Feb 4. Exp Eye Res. 2020. PMID: 32032628 Free PMC article. Review.
-
Biocidal efficacy of multipurpose solutions against Gram-negative organisms associated with corneal infiltrative events.Clin Exp Optom. 2017 Jul;100(4):357-364. doi: 10.1111/cxo.12509. Epub 2017 Feb 14. Clin Exp Optom. 2017. PMID: 28194876 Free PMC article.
References
-
- Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–82. - PubMed
-
- Hazlett LD. Bacterial infections of the cornea (Pseudomonas aeruginosa) Chem Immunol Allergy. 2007;92:185–94. - PubMed
-
- Pearlman E, Gillette-ferguson I. Onchocerca volvulus, Wolbachia and river blindness. Chem Immunol Allergy. 2007;92:254–65. - PubMed
-
- Hume EB, Dajcs JJ, Moreau JM, et al. Staphylococcus corneal virulence in a new topical model of infection. Invest Ophthalmol Vis Sci. 2001;42:2904–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

