Consequences of unlocking the cardiac myosin molecule in human myocarditis and cardiomyopathies
- PMID: 18781470
- PMCID: PMC3108569
- DOI: 10.1080/08916930802031579
Consequences of unlocking the cardiac myosin molecule in human myocarditis and cardiomyopathies
Abstract
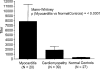
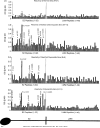
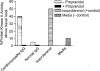
Myocarditis, often initiated by viral infection, may progress to autoimmune inflammatory heart disease, dilated cardiomyopathy and heart failure. Although cardiac myosin is a dominant autoantigen in animal models of myocarditis and is released from the heart during viral myocarditis, the characterization, role and significance of anti-cardiac myosin autoantibodies is poorly defined. In our study, we define the human cardiac myosin epitopes in human myocarditis and cardiomyopathies and establish a mechanism to explain how anti-cardiac myosin autoantibodies may contribute to heart disease. We show that autoantibodies to cardiac myosin in sera from myocarditis and dilated cardiomyopathies in humans targeted primarily epitopes in the S2 hinge region of cardiac myosin. In addition, anti-cardiac myosin antibodies in sera or purified IgG from myocarditis and cardiomyopathy targeted the beta-adrenergic receptor and induced antibody-mediated cAMP-dependent protein kinase A (PKA) cell signaling activity in heart cells. Antibody-mediated PKA activity in sera was abrogated by absorption with anti-human IgG. Antibody-mediated cell signaling of PKA was blocked by antigen-specific inhibition by human cardiac myosin or the beta-adrenergic receptor but not the alpha adrenergic receptor or bovine serum albumin. Propranolol, a beta blocker and inhibitor of the beta-adrenergic receptor pathway also blocked the antibody-mediated signaling of the beta-adrenergic receptor and PKA. The data suggest that IgG antibody against human cardiac myosin reacts with the beta-adrenergic receptor and triggers PKA signaling in heart cells. In summary, we have identified a new class of crossreactive autoantibodies against human cardiac myosin and the beta-adrenergic receptor in the heart. In addition, we have defined disease specific peptide epitopes in the human cardiac myosin rod S2 region in human myocarditis and cardiomyopathy as well as a mechanistic role of autoantibody in the pathogenesis of disease.
Figures




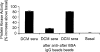
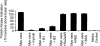
References
-
- Caforio ALP, Goldman JH, Haven AJ, Baig KM, McKenna WJ. Evidence for autoimmunity to myosin and other heart-specific autoantigens in patients with dilated cardiomyopathy and their relatives. Int J Cardiol. 1996;54:157–163. - PubMed
-
- Felker GM, Hu W, Hare JM, Hruban RH, Baughman KI, Kasper EK. The spectrum of dilated cardiomyopathy. The Johns Hopkins experience with 1278 patients. Medicine. 1999;78:270–283. - PubMed
-
- Aretz HT. Myocarditis: The Dallas criteria. Hum Pathol. 1987;18:619–624. - PubMed
-
- Aretz HT, Billingham ME, Edwards WD, Factor SM, Fallon JT, Fenoglio JJ, Jr, et al. Myocarditis. A histopathologic definition and classification. Am J Cardiovasc Pathol. 1987;1:3–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
