Recombinant cardiac myosin fragment induces experimental autoimmune myocarditis via activation of Th1 and Th17 immunity
- PMID: 18781477
- PMCID: PMC2702149
- DOI: 10.1080/08916930802167902
Recombinant cardiac myosin fragment induces experimental autoimmune myocarditis via activation of Th1 and Th17 immunity
Abstract
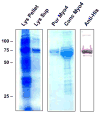
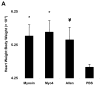
The specificity and function of T helper (Th) immune responses underlying the induction, progression, and resolution of experimental autoimmune myocarditis (EAM) in A/J mice are unclear. Published data suggest involvement of both Th1 and Th2 responses in EAM; however, the previous inability to assess antigen-specific in vivo and in vitro T-cell responses in cardiac myosin-immunized animals has confounded our understanding of this important model of autoimmune myocarditis. The goal of our study was to develop an alternative model of EAM based on a recombinant fragment of cardiac myosin, in hopes that the recombinant protein will permit measurement of functional T-cell responses that is not possible with purified native protein. A/J mice immunized with a recombinant fragment of cardiac myosin spanning amino acids 1074-1646, termed Myo4, developed severe myocarditis characterized by cardiac hypertrophy, massive mononuclear cell infiltration and fibrosis, three weeks post-immunization. The mice also developed an IgG1 dominant humoral immune response specific for both Myo4 and purified cardiac myosin. The in vitro stimulation of splenocytes harvested from Myo4-immunized animals with Myo4 resulted in cellular proliferation with preferential production of the Th1- and Th17-associated cytokines, IFN-gamma, IL-17, and IL-6, respectively. Production of IL-4 was negligible by comparison. This study describes a new model of EAM, inducible by immunization with a specific fragment of cardiac myosin, from which antigen-specific analyses reveal an importance for both Th1 and Th17 immunity.
Figures


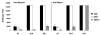

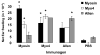
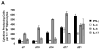
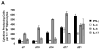
Similar articles
-
[Immune state of Th1, Th2 and Th17 subpopulation in experimental autoimmune myocarditis].Sichuan Da Xue Xue Bao Yi Xue Ban. 2011 Nov;42(6):751-6. Sichuan Da Xue Xue Bao Yi Xue Ban. 2011. PMID: 22332535 Chinese.
-
Apigenin Attenuates Experimental Autoimmune Myocarditis by Modulating Th1/Th2 Cytokine Balance in Mice.Inflammation. 2016 Apr;39(2):678-86. doi: 10.1007/s10753-015-0294-y. Inflammation. 2016. PMID: 26658748
-
Recombinant murine interleukin-12 facilitates induction of cardiac myosin-specific type 1 helper T cells in rats.Circ Res. 1998 Jun 1;82(10):1035-42. doi: 10.1161/01.res.82.10.1035. Circ Res. 1998. PMID: 9622156
-
Cardiac myosin and the TH1/TH2 paradigm in autoimmune myocarditis.Am J Pathol. 2001 Jul;159(1):5-12. doi: 10.1016/S0002-9440(10)61665-3. Am J Pathol. 2001. PMID: 11438446 Free PMC article. Review. No abstract available.
-
Pathogenesis of myocarditis and dilated cardiomyopathy.Adv Immunol. 2008;99:95-114. doi: 10.1016/S0065-2776(08)00604-4. Adv Immunol. 2008. PMID: 19117533 Review.
Cited by
-
Th17 cells facilitate the humoral immune response in patients with acute viral myocarditis.J Clin Immunol. 2010 Mar;30(2):226-34. doi: 10.1007/s10875-009-9355-z. Epub 2009 Dec 10. J Clin Immunol. 2010. PMID: 20012175
-
T cells in cardiac health and disease.J Clin Invest. 2025 Jan 16;135(2):e185218. doi: 10.1172/JCI185218. J Clin Invest. 2025. PMID: 39817455 Free PMC article. Review.
-
Heat-killed Trypanosoma cruzi induces acute cardiac damage and polyantigenic autoimmunity.PLoS One. 2011 Jan 21;6(1):e14571. doi: 10.1371/journal.pone.0014571. PLoS One. 2011. PMID: 21283741 Free PMC article.
-
IgG2 rules: N-acetyl-β-D-glucosamine-specific IgG2 and Th17/Th1 cooperation may promote the pathogenesis of acute rheumatic heart disease and be a biomarker of the autoimmune sequelae of Streptococcus pyogenes.Front Cardiovasc Med. 2023 Feb 6;9:919700. doi: 10.3389/fcvm.2022.919700. eCollection 2022. Front Cardiovasc Med. 2023. PMID: 36815140 Free PMC article.
-
Th17-related cytokines in systemic lupus erythematosus patients with dilated cardiomyopathies: a possible linkage to parvovirus B19 infection.PLoS One. 2014 Dec 2;9(12):e113889. doi: 10.1371/journal.pone.0113889. eCollection 2014. PLoS One. 2014. PMID: 25462010 Free PMC article.
References
-
- Pisani B, Taylor DO, Mason JW. Inflammatory myocardial diseases and cardiomyopathies. Am J Med. 1997;102:459–469. - PubMed
-
- Caforio AL, McKenna WJ. Recognition and optimum management of myocarditis. Drugs. 1996;52:515–525. - PubMed
-
- Feldman AM, McNamara D. Myocarditis. N Engl J Med. 2000;343:1388–1398. - PubMed
-
- Latif N, Baker CS, Dunn MJ, Rose ML, Brady P, Yacoub MH. Frequency and specificity of antiheart antibodies in patients with dilated cardiomyopathy detected using SDS-PAGE and western blotting. J Am Coll Cardiol. 1993;22:1378–1384. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
