Insights into directing group ability in palladium-catalyzed C-H bond functionalization
- PMID: 18781752
- PMCID: PMC2733373
- DOI: 10.1021/ja8045519
Insights into directing group ability in palladium-catalyzed C-H bond functionalization
Abstract
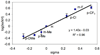
This paper describes a detailed investigation of factors controlling the dominance of a directing group in Pd-catalyzed ligand-directed arene acetoxylation. Mechanistic studies, involving reaction kinetics, Hammett analysis, kinetic isotope effect experiments, and the kinetic order in oxidant, have been conducted for a series of different substrates. Initial rates studies of substrates bearing different directing groups showed that these transformations are accelerated by the use of electron-withdrawing directing groups. However, in contrast, under conditions where two directing groups are in competition with one another in the same reaction flask, substrates with electron-donating directing groups react preferentially. These results are discussed in the context of the proposed mechanism for Pd-catalyzed arene acetoxylation.
Figures
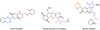










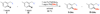
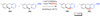


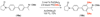

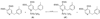
References
-
- Hull KL, Sanford MS. J. Am. Chem. Soc. 2007;129:11904. - PubMed
- Hull KL, Lanni EL, Sanford MS. J. Am. Chem. Soc. 2006;128:14047. - PubMed
- Hull KL, Anani WQ, Sanford MS. J. Am. Chem. Soc. 2006;128:7134. - PubMed
- Kalyani D, Dick AR, Anani WQ, Sanford MS. Tetrahedron. 2006;62:11483. - PubMed
- Kalyani D, Dick AR, Anani WQ, Sanford MS. Org. Lett. 2006;8:2523. - PubMed
- Desai LV, Malik HA, Sanford MS. Org. Lett. 2006;8:1141. - PubMed
- Kalyani D, Sanford MS. Org. Lett. 2005;7:4149. - PubMed
- Kalyani D, Deprez NR, Desai LV, Sanford MS. J. Am. Chem. Soc. 2005;127:7330. - PubMed
- Desai LV, Hull KL, Sanford MS. J. Am. Chem. Soc. 2004;126:2300. - PubMed
- Dick AR, Hull KL, Sanford MS. J. Am. Chem. Soc. 2004;126:2300. - PubMed
-
-
For selected examples of palladium-catalyzed directed C–H activation/C–heteroatom bond forming reactions, see: Inamoto K, Saito T, Katsuno M, Sakamoto T, Hiroya K. Org. Lett. 2007;9:2931. Thu HY, Yu WY, Che CM. J. Am. Chem. Soc. 2006;128:9048. Wan X, Ma Z, Li B, Zhang K, Cao S, Zhang S, Shi Z. J. Am. Chem. Soc. 2006;128:7416. Reddy BVS, Reddy LR, Corey E. J. Org. Lett. 2006;8:3391. Wang D, Hao X, Wu D, Yu JQ. Org. Lett. 2006;8:3387. Yu JQ, Giri R, Chen X. Org. Biomol. Chem. 2006;4:4041. and references therein. Tsang WCP, Zheng N, Buchwald SL. J. Am. Chem. Soc. 2005;127:14560. Giri R, Liang J, Lei JG, Li JJ, Wang DH, Chen X, Naggar IC, Guo C, Foxman BM, Yu JQ. Angew. Chem., Int. Ed. 2005;44:7420. Giri R, Chen X, Yu JQ. Angew. Chem., Int. Ed. 2005;44:2112. Dangel BD, Johnson JA, Sames D. J. Am. Chem. Soc. 2001;123:8149.
-
-
-
For selected examples of palladium-catalyzed directed C–H activation/C–C bond forming reactions, see: Yu W-Y, Sit WN, Lai K-M, Zhou Z, Chan ASC. J. Am. Chem. Soc. 2008;130:3304. Beccalli EM, Broggini G, Martinelli M, Sottocornola S. Chem. Rev. 2007;107:5318. Alberico D, Scott ME, Lautens M. Chem. Rev. 2007;107:174. Cai G, Fu Y, Li Y, Wan X, Shi Z. J. Am. Chem. Soc. 2007;129:7666. Giri R, Maugel NL, Li JJ, Wang DH, Breazzano SP, Saunders LB, Yu JQ. J. Am. Chem. Soc. 2007;129:3510. Xia J-B, You S-L. Organometallics. 2007;26:4869. Chiong HA, Daugulis O. Org. Lett. 2007;9:1449. Shi ZJ, Li B, Wan X, Cheng J, Fang Z, Cao B, Qin C, Wang Y. Angew. Chem., Int. Ed. 2007;46:5554. Chen X, Goodhue CE, Yu JQ. J. Am. Chem. Soc. 2006;128:12634. Chen X, Li JJ, Hao XS, Goodhue CE, Yu JQ. J. Am. Chem. Soc. 2006;128:78. Orito K, Miyazawa M, Nakamura T, Horibata A, Ushito H, Nagasaki H, Yuguchi M, Yamashita S, Yamazaki T, Tokuda M. J. Org. Chem. 2006;71:5951. Zaitsev VG, Shabashov D, Daugulis O. J. Am. Chem. Soc. 2005;127:13154. Zaitsev VG, Daugulis O. J. Am. Chem. Soc. 2005;127:4156. Shabashov D, Daugulis O. Org. Lett. 2005;7:3657. Daugulis O, Zaitsev VG. Angew. Chem., Int. Ed. 2005;44:4046. Orito K, Horibata A, Nakamura T, Ushito H, Nagasaki H, Yuguchi M, Yamashita S, Tokuda M. J. Am. Chem. Soc. 2004;126:14342. Wakui H, Kawasaki S, Satoh T, Miura M, Nomura M. J. Am. Chem. Soc. 2004;126:8658. Hennessy EJ, Buchwald SL. J. Am. Chem. Soc. 2003;125:12084. Ritleng V, Sirlin C, Pfeffer M. Chem. Rev. 2002;102:1731. Boele MDK, van Strijdonck GPF, de Vries AHM, Kamer PCJ, de Vries JG, van Leeuwen PWNM. J. Am. Chem. Soc. 2002;124:1586. Dyker G. Angew. Chem., Int. Ed. Engl. 1999;38:1698. Tremont SJ, Rahman HU. J. Am. Chem. Soc. 1984;106:5759.
-
-
- Ali A, Hunt JA, Kallashi F, Kowalchick JE, Kim D, Smith CJ, Sinclair PJ, Sweis RF, Taylor GE, Thompson CF, Chen L, Quraishi N. PCT Int. Appl. 2007 WO 2007070173.
- Zhou P, Bernotas RC, Li Y, Nowak PW, Cole DC, Manas ES, Fan Y, Yan Y. PCT Int. Appl. 2007 WO 2007078813.
- Chen J, Deady LW, Kaye AJ, Finlay GJ, Baguley BC, Denny WA. Bioorg. Med. Chem. 2002;10:2381. - PubMed
-
-
For previous examples of the stoichiometric cyclopalladation of benzylpyridine, see: Mawo RY, Johnson DM, Wood JL, Smoliakova IP. J. Organomet. Chem. 2008;693:33. and references therein. Hiraki K, Fuchita Y, Takechi K. Inorg. Chem. 1981;20:4316.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

